Question 9- Solve the question based off of this information. Liquid werewolf blood boils at 32.0 °A and freezes at -12.0 °A. Its heat of fusion is 65.0 J/g and heat of vaporization is 400. J/g. The specific heat of liquid werewolf blood is 1.927 J/g°A. Look at attached images for conversion factor.
In the game dwarves and elves measure temperature differently. Dwarves measure in units of degrees Vacius (°V) and elves measure in units of degrees Arwenheit (°A). The following is used to convert between the two:
TA =TV + 54/ 3.1
Consider the numbers 3.1 and 53 to be exact by definition.
What is the heat released in J when 0.294 kg of liquid werewolf blood at 24.0 °A is cooled to -8.0 °A?
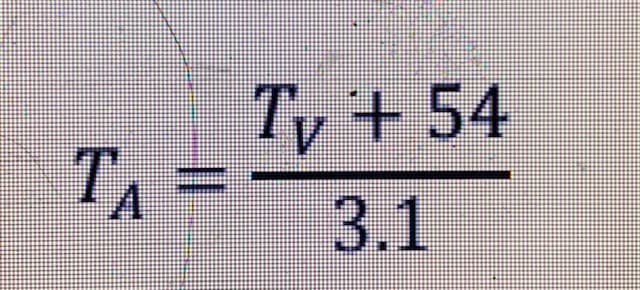
Given Information-
- Boiling point of liquid werewolf blood= 32.0 oA
- Freezing point of liquid werewolf blood =-12 oA
- heat of fusion = 65.0 J/g
- heat of vaporization= 400 J/g
- specific heat of liquid werewolf blood = 1.927 J/g oA
- mass of werewolf blood = 0.294 kg
- initial temperature = 24o A
- final temperature = -8o A
- heat released in J (q) = ?
mass of liquid werewolf blood = 0.294 kg
Conversion factor between kg and g is 1 kg = 1000 g
Start with 0.624 kg and arrange the given conversion factor to convert mass into grams.
0.294 kg * = 294 g
Step by step
Solved in 3 steps






