Use the diagram below to answer numbers 5-6 Solid Liquid Gas 5. Which of these conclusions can be derived from the given diagram of the phases of matter? * a. Liquids have definite shape and definite volume. b. Molecules in gases have less energy than solid. C. Molecules of solids are more compact than liquids. d. Only molecules of solids have the ability to scatter in a medium. 6. Based on the above diagram, which phase will the molecules have the least kinetic energy? * а. Gas b. Liquid C. Solid d. Both solid and liquid 7. Which of the following is NOT true about the Particle Theory of Matter? * a. All matter is made up of particles. b. All particles of one substance are different. C. The particles of matter are in constant motion. d. Ther are intermo lar spaces between particles. 8. What property of air is shown when a bottle was filled with air and sealed with a
Use the diagram below to answer numbers 5-6 Solid Liquid Gas 5. Which of these conclusions can be derived from the given diagram of the phases of matter? * a. Liquids have definite shape and definite volume. b. Molecules in gases have less energy than solid. C. Molecules of solids are more compact than liquids. d. Only molecules of solids have the ability to scatter in a medium. 6. Based on the above diagram, which phase will the molecules have the least kinetic energy? * а. Gas b. Liquid C. Solid d. Both solid and liquid 7. Which of the following is NOT true about the Particle Theory of Matter? * a. All matter is made up of particles. b. All particles of one substance are different. C. The particles of matter are in constant motion. d. Ther are intermo lar spaces between particles. 8. What property of air is shown when a bottle was filled with air and sealed with a
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 14CR
Related questions
Question
Answer number 5 to 8 pls. I don't want to waste my money here.
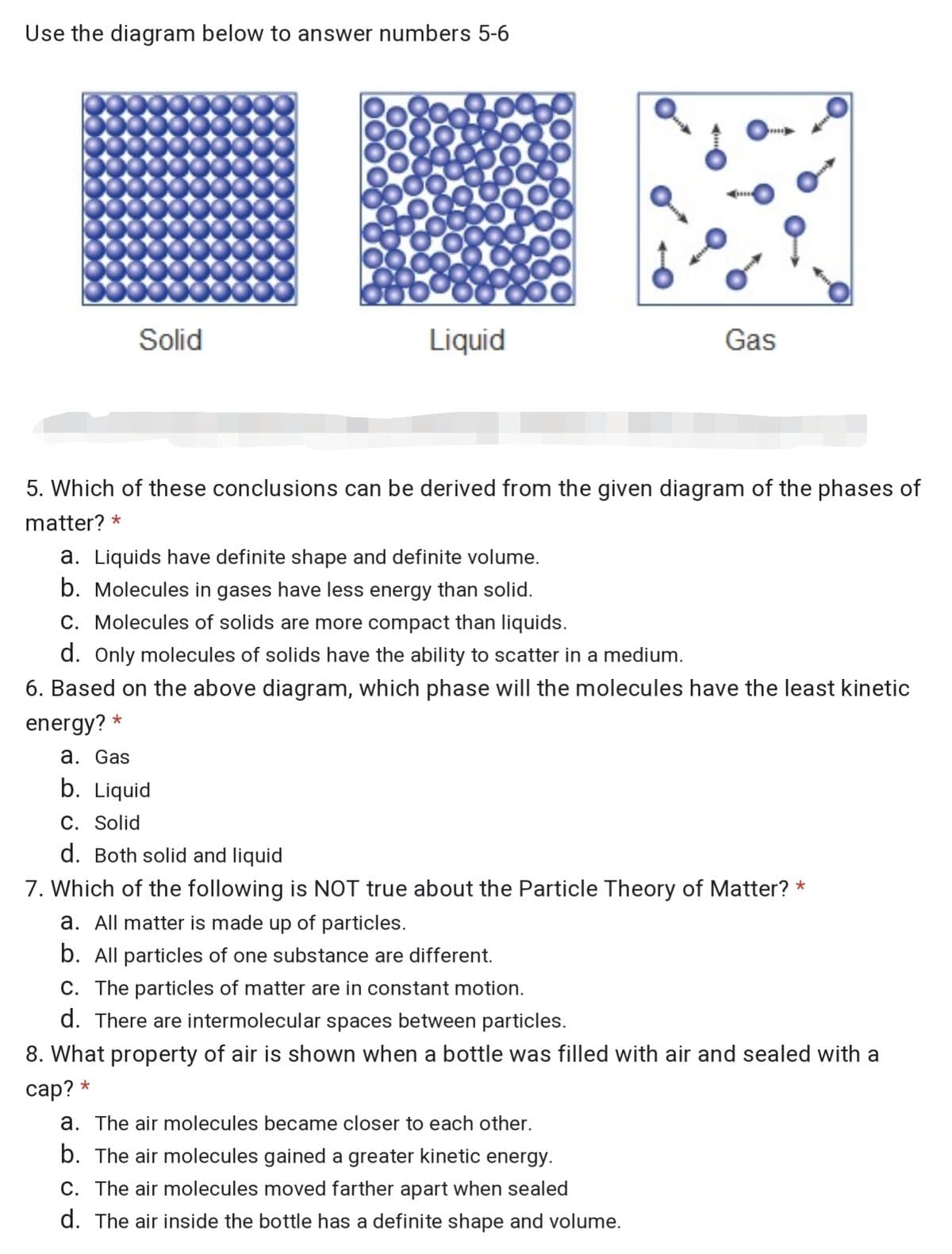
Transcribed Image Text:Use the diagram below to answer numbers 5-6
Solid
Liquid
Gas
5. Which of these conclusions can be derived from the given diagram of the phases of
matter? *
a. Liquids have definite shape and definite volume.
b. Molecules in gases have less energy than solid.
C. Molecules of solids are more compact than liquids.
d. Only molecules of solids have the ability to scatter in a medium.
6. Based on the above diagram, which phase will the molecules have the least kinetic
energy? *
a. Gas
b. Liquid
C. Solid
d. Both solid and liquid
7. Which of the following is NOT true about the Particle Theory of Matter? *
a. All matter is made up of particles.
b. All particles of one substance are different.
C. The particles of matter are in constant motion.
d. There are intermolecular spaces between particles.
8. What property of air is shown when a bottle was filled with air and sealed with a
саp? *
a. The air molecules became closer to each other.
b. The air molecules gained a greater kinetic energy.
C. The air molecules moved farther apart when sealed
d. The air inside the bottle has a definite shape and volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning