u are provided sample solutions containing the following compounds, but in each case, there is some unknown interference that prevents you from making accurate absorbanc fault wavelength in the simulation (the "preset" wavelength) corresponds to Amax , as discussed above.) se the variable radio button in the simulation to identify whether there are any other wavelengths that correspond to local maxima such that they could be used to determine con ternative wavelength (if any) for each molecule It is easiest to see changes in the absorbance when you use a high analyte concentration. ag the appropriate alternative wavelengths to their respective targets. If there are no appropriate wavelength values, drag no additional peaks to that target. View Available Hint(s) Reset Help no additional peaks 380 nm 549 nm 544 nm 392 nm 433 nm 411 nm 761 nm Co(NO,), KCr,O, CoCl, K,CrO, NiCl, KMNO,
u are provided sample solutions containing the following compounds, but in each case, there is some unknown interference that prevents you from making accurate absorbanc fault wavelength in the simulation (the "preset" wavelength) corresponds to Amax , as discussed above.) se the variable radio button in the simulation to identify whether there are any other wavelengths that correspond to local maxima such that they could be used to determine con ternative wavelength (if any) for each molecule It is easiest to see changes in the absorbance when you use a high analyte concentration. ag the appropriate alternative wavelengths to their respective targets. If there are no appropriate wavelength values, drag no additional peaks to that target. View Available Hint(s) Reset Help no additional peaks 380 nm 549 nm 544 nm 392 nm 433 nm 411 nm 761 nm Co(NO,), KCr,O, CoCl, K,CrO, NiCl, KMNO,
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 43P
Related questions
Question
Need help on the following please.
![You are provided sample solutions containing the following compounds, but in each case, there is some unknown interference that prevents you from making accurate absorbance measurements at their optimal wavelengths (Amax). [Recall that the
default wavelength in the simulation (the "preset" wavelength) corresponds to Amax, as discussed above.]
Use the variable radio button in the simulation to identify whether there are any other wavelengths that correspond to local maxima such that they could be used to determine concentration, then complete the diagram below by labeling the
alternative wavelength (if any) for each molecule. It is easiest to see changes in the absorbance when you use a high analyte concentration.
Drag the appropriate alternative wavelengths to their respective targets. If there are no appropriate wavelength values, drag no additional peaks to that target.
• View Available Hint(s)
Reset
Help
no additional
peaks
380 nm
549 nm
544 nm
392 nm
433 nm
411 nm.
761 nm
Co(NO,),
K,Cr,O,
COCI,
K,CrO,
NICI,
KMNO,](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F064a43ae-0fdb-4c56-97b0-db6fb6dece57%2Fc10ebbcc-c063-444a-8282-f80dfa4d16d6%2Fvk7eu7_processed.png&w=3840&q=75)
Transcribed Image Text:You are provided sample solutions containing the following compounds, but in each case, there is some unknown interference that prevents you from making accurate absorbance measurements at their optimal wavelengths (Amax). [Recall that the
default wavelength in the simulation (the "preset" wavelength) corresponds to Amax, as discussed above.]
Use the variable radio button in the simulation to identify whether there are any other wavelengths that correspond to local maxima such that they could be used to determine concentration, then complete the diagram below by labeling the
alternative wavelength (if any) for each molecule. It is easiest to see changes in the absorbance when you use a high analyte concentration.
Drag the appropriate alternative wavelengths to their respective targets. If there are no appropriate wavelength values, drag no additional peaks to that target.
• View Available Hint(s)
Reset
Help
no additional
peaks
380 nm
549 nm
544 nm
392 nm
433 nm
411 nm.
761 nm
Co(NO,),
K,Cr,O,
COCI,
K,CrO,
NICI,
KMNO,
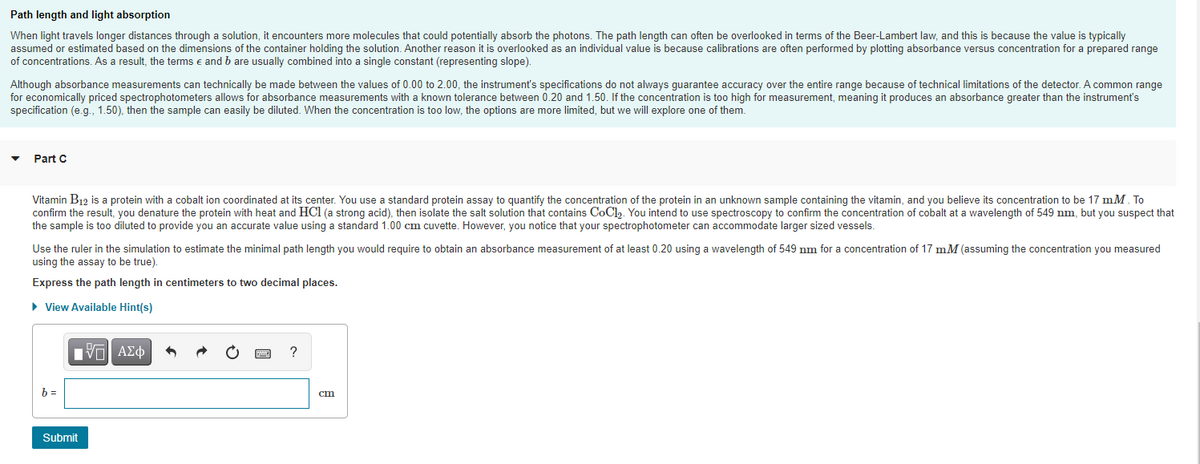
Transcribed Image Text:Path length and light absorption
When light travels longer distances through a solution, it encounters more molecules that could potentially absorb the photons. The path length can often be overlooked in terms of the Beer-Lambert law, and this is because the value is typically
assumed or estimated based on the dimensions of the container holding the solution. Another reason it is overlooked as an individual value is because calibrations are often performed by plotting absorbance versus concentration for a prepared range
of concentrations. As a result, the terms e and b are usually combined into a single constant (representing slope).
Although absorbance measurements can technically be made between the values of 0.00 to 2.00, the instrument's specifications do not always guarantee accuracy over the entire range because of technical limitations of the detector. A common range
for economically priced spectrophotometers allows for absorbance measurements with a known tolerance between 0.20 and 1.50. If the concentration is too high for measurement, meaning it produces an absorbance greater than the instrument's
specification (e.g., 1.50), then the sample can easily be diluted. When the concentration is too low, the options are more limited, but we will explore one of them.
Part C
Vitamin B12 is a protein with a cobalt ion coordinated at its center. You use a standard protein assay to quantify the concentration of the protein in an unknown sample containing the vitamin, and you believe its concentration to be 17 mM. To
confirm the result, you denature the protein with heat and HCl (a strong acid), then isolate the salt solution that contains CoCl. You intend to use spectroscopy to confirm the concentration of cobalt at a wavelength of 549 nm, but you suspect that
the sample is too diluted to provide you an accurate value using a standard 1.00 cm cuvette. However, you notice that your spectrophotometer can accommodate larger sized vessels.
Use the ruler in the simulation to estimate the minimal path length you would require to obtain an absorbance measurement of at least 0.20 using a wavelength of 549 nm for a concentration of 17 mM (assuming the concentration you measured
using the assay to be true).
Express the path length in centimeters to two decimal places.
• View Available Hint(s)
?
b =
cm
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,