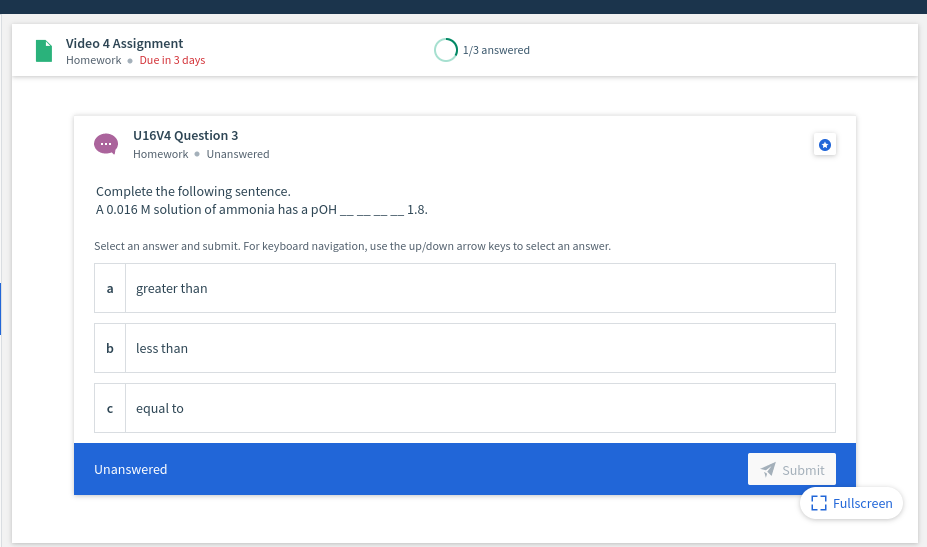
U16V4 Question 3 Homework • Unanswered Complete the following sentence. A 0.016 M solution of ammonia has a pOH 1.8. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
U16V4 Question 3 Homework • Unanswered Complete the following sentence. A 0.016 M solution of ammonia has a pOH 1.8. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 58P
Related questions
Question
Please answer correctly. Thank you.
Transcribed Image Text:Video 4 Assignment
1/3 answered
Homework Due in 3 days
U16V4 Question 3
Homework • Unanswered
Complete the following sentence.
A 0.016 M solution of ammonia has a pOH,
1.8.
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
a
greater than
less than
equal to
Unanswered
Submit
{ Fullscreen
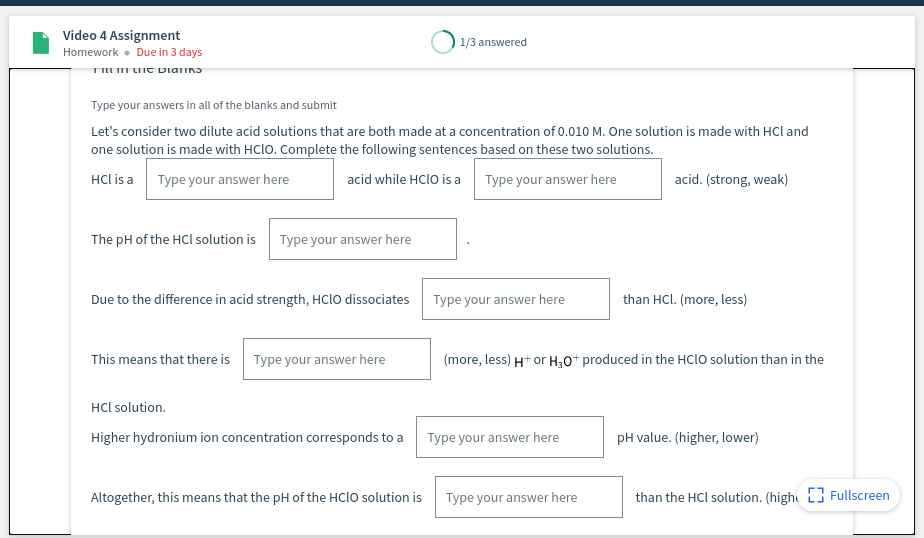
Transcribed Image Text:Video 4 Assignment
1/3 answered
Homework • Due in 3 days
Type your answers in all of the blanks and submit
Let's consider two dilute acid solutions that are both made at a concentration of 0.010 M. One solution is made with HCI and
one solution is made with HCIO. Complete the following sentences based on these two solutions.
HCl is a
Type your answer here
acid while HClo is a
Type your answer here
acid. (strong, weak)
The pH of the HCl solution is
Type your answer here
Due to the difference in acid strength, HCIO dissociates
Type your answer here
than HCl. (more, less)
This means that there is Type your answer here
(more, less) H+ or H30+ produced in the HCIO solution than in the
HCl solution.
Higher hydronium ion concentration corresponds to a
Type your answer here
pH value. (higher, lower)
Altogether, this means that the pH of the HClo solution is
Type your answer here
than the HCl solution. (hight 3 Fullscreen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning