UESTION 2 Which answer correctly predicts the multiplicity (the number of peaks as a result of splitting) for each shaded proton in the (Assume the sample contains a trace of an acidic or basic impurity and is not ultrapure) HgC-C-OH Ha = 4, Hb = 1, Hc = 2 0 Ha 8, Hb = 2, Hc = 2 Ha 7, Hb = 1, Hc = 2 Ha = 5, Hb = 2, Hc = 2
UESTION 2 Which answer correctly predicts the multiplicity (the number of peaks as a result of splitting) for each shaded proton in the (Assume the sample contains a trace of an acidic or basic impurity and is not ultrapure) HgC-C-OH Ha = 4, Hb = 1, Hc = 2 0 Ha 8, Hb = 2, Hc = 2 Ha 7, Hb = 1, Hc = 2 Ha = 5, Hb = 2, Hc = 2
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.34QAP
Related questions
Question
Transcribed Image Text:u/webapps/assessment/take/launch.jsp?course_assessment_id%3_148362_1&course_id%3D_147285_1&content_id%3D
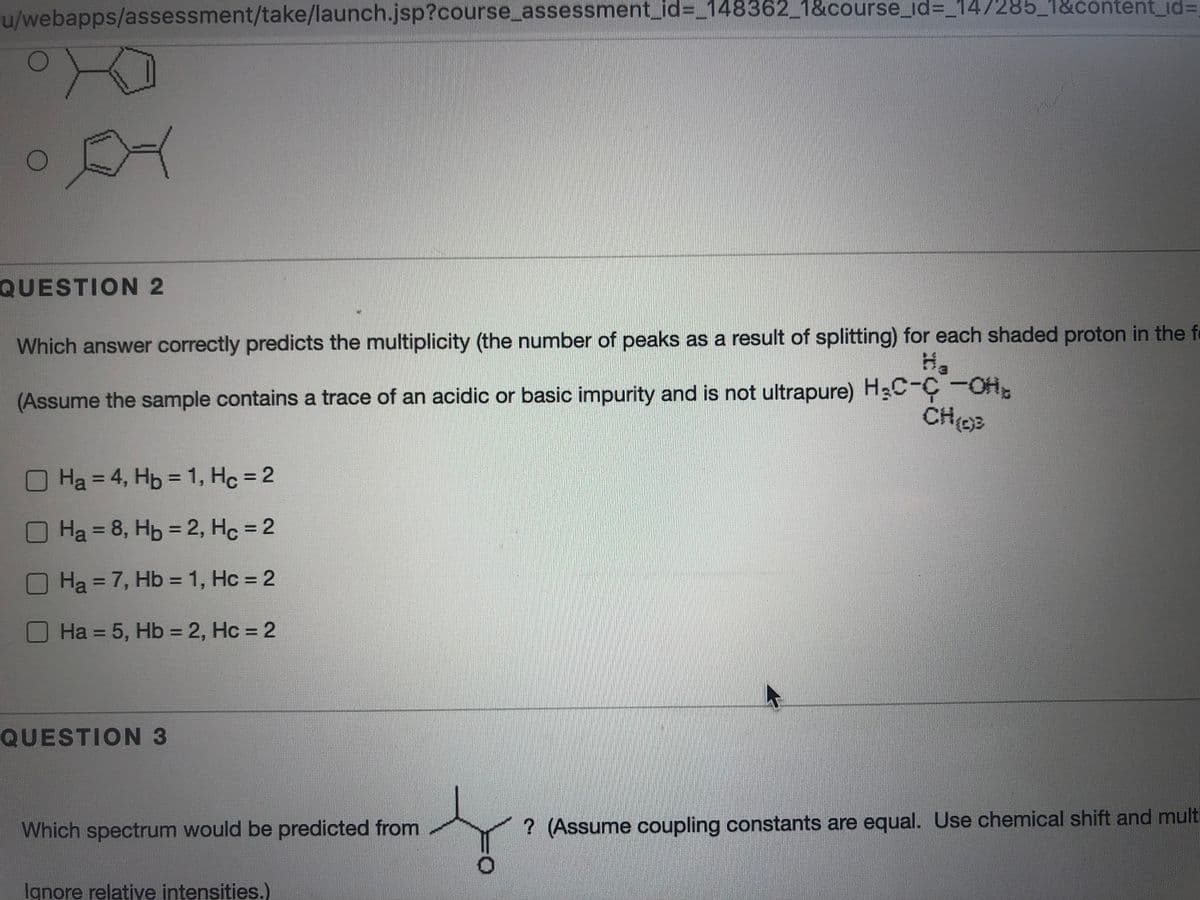
QUESTION 2
Which answer correctly predicts the multiplicity (the number of peaks as a result of splitting) for each shaded proton in the fe
(Assume the sample contains a trace of an acidic or basic impurity and is not ultrapure) H3C-C-OH.
Ha = 4, Hp = 1, Hc = 2
%3D
%3D
OHa = 8, Hb = 2, Hc = 2
%3D
%3D
OHa=7, Hb = 1, Hc = 2
%3D
%3D
%3D
O Ha = 5, Hb = 2, Hc = 2
%3D
QUESTION 3
Which spectrum would be predicted from
? (Assume coupling constants are equal. Use chemical shift and multi
Ignore relative intensities.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

