EDTA is a hexaprotic system with the pK, values: Fooc-CH2 H,C-coo pKal = 0.00, pK2 = 1.50, pKas = 2.00, pK4 = 2.69, N-CH2-CH2-N: pKus = 6.13, and pKu6 = 10.37. The distribution of the various protonated forms of EDTA "ooc-CH, H,C-coo EDTA (or Y4-) will therefore vary with pH. For equilibrium calculations involving metal complexes with EDTA, it is convenient to calculate the fraction of EDTA that is in the completely unprotonated form, Y*-. This fraction is designated ay4-. Calculate ayt- at two pH values. pH = 3.70 ay4- = pH = 10.45 ay4- =
EDTA is a hexaprotic system with the pK, values: Fooc-CH2 H,C-coo pKal = 0.00, pK2 = 1.50, pKas = 2.00, pK4 = 2.69, N-CH2-CH2-N: pKus = 6.13, and pKu6 = 10.37. The distribution of the various protonated forms of EDTA "ooc-CH, H,C-coo EDTA (or Y4-) will therefore vary with pH. For equilibrium calculations involving metal complexes with EDTA, it is convenient to calculate the fraction of EDTA that is in the completely unprotonated form, Y*-. This fraction is designated ay4-. Calculate ayt- at two pH values. pH = 3.70 ay4- = pH = 10.45 ay4- =
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter19: Carboxylic Anhydrides, Esters, And Amides
Section: Chapter Questions
Problem 19.6P
Related questions
Question
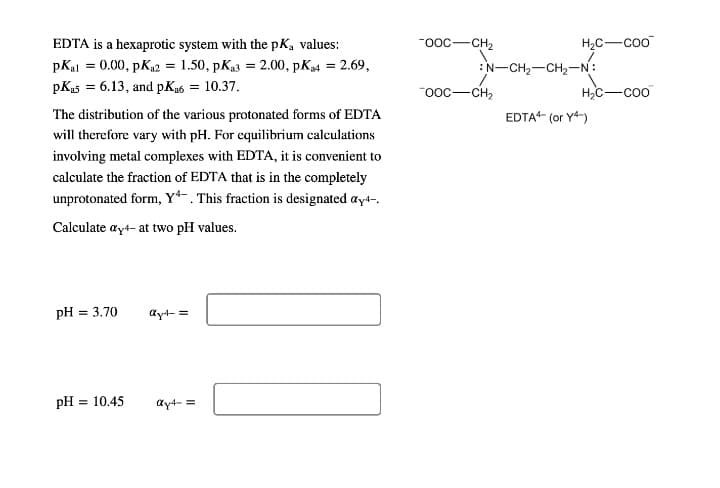
Transcribed Image Text:EDTA is a hexaprotic system with the pK, values:
-ooc-CH2
H2C-coo
pKal = 0.00, pKa2 = 1.50, pKa3 = 2.00, pK4 = 2.69,
:N-CH2-CH2-N:
pKas = 6.13, and pKa6 = 10.37.
"ooc-CH2
H,C-coo
The distribution of the various protonated forms of EDTA
EDTA (or Y4-)
will therefore vary with pH. For equilibrium calculations
involving metal complexes with EDTA, it is convenient to
calculate the fraction of EDTA that is in the completely
unprotonated form, Y*-. This fraction is designated ayt-,
Calculate ayt- at two pH values.
pH = 3.70
ayt- =
pH = 10.45
ay4- =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning