Gaseous butane (CH, (CH,) CH,) reacts with gaseous oxygen gas (O2) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). What is the theoretical yield of water formed from the reaction of 3.5 g of butane and 4.4 g of oxygen gas? Round your answer to 2 significant figures.
Gaseous butane (CH, (CH,) CH,) reacts with gaseous oxygen gas (O2) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). What is the theoretical yield of water formed from the reaction of 3.5 g of butane and 4.4 g of oxygen gas? Round your answer to 2 significant figures.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 19PS
Related questions
Question
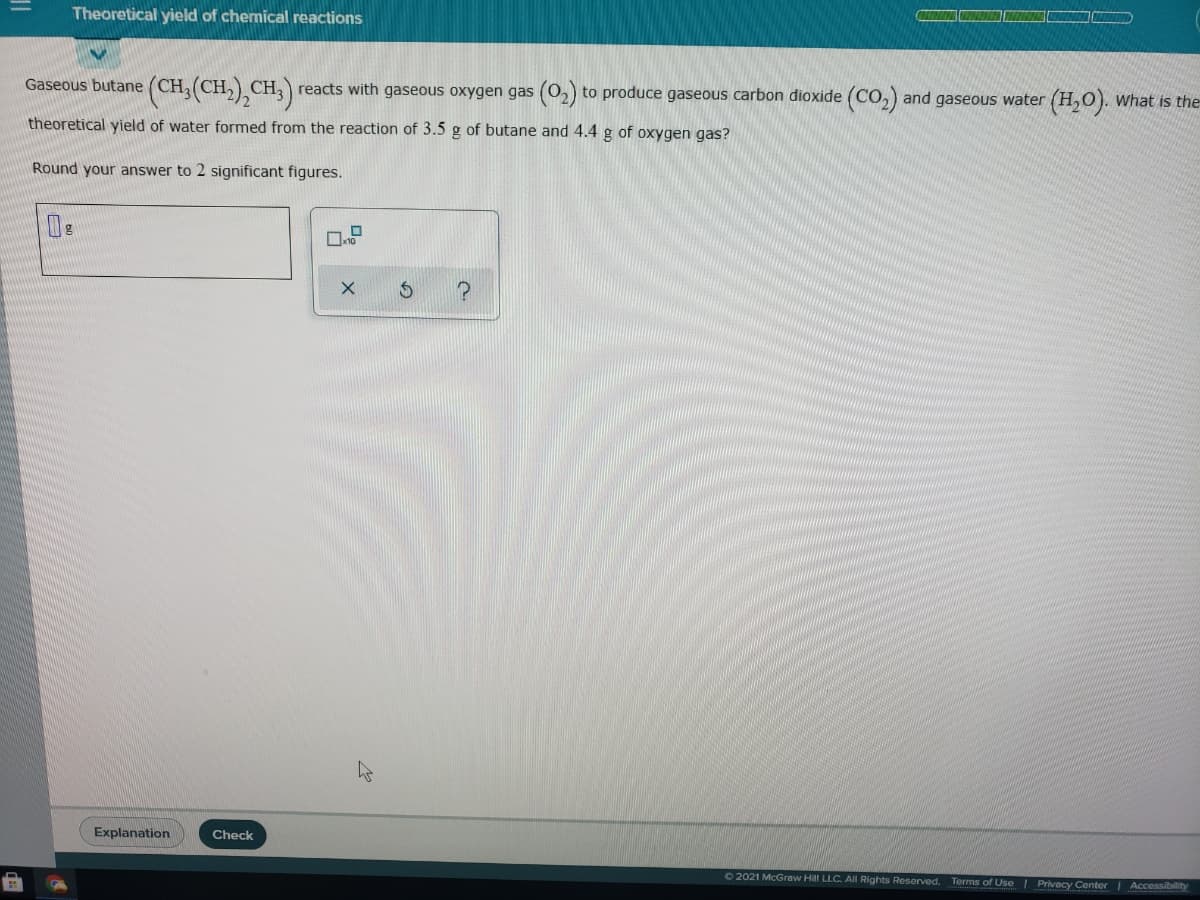
Transcribed Image Text:Theoretical yield of chemical reactions
Gaseous butane (CH, (CH
,) CH3) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (Co,) and gaseous water (H,O). What is the
theoretical yield of water formed from the reaction of 3.5 g of butane and 4.4 g of oxygen gas?
Round your answer to 2 significant figures.
Explanation
Check
O 2021 McGraw Hill LLC. All Rights Reserved.
Terms of Use | Privacy Center Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div