Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. H3C H₂C a O ao H₂ b b Hc N CH₂ Submit Answer OC₂H5 The number of unshared pairs at atom a is The number of unshared pairs at atom bis The number of unshared pairs at atom cis The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom cis v Retry Entire Group 9 more group attempts remaining V 0 1 2 3 4 Previous Email Instructor Next Save and Exit
Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. H3C H₂C a O ao H₂ b b Hc N CH₂ Submit Answer OC₂H5 The number of unshared pairs at atom a is The number of unshared pairs at atom bis The number of unshared pairs at atom cis The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom cis v Retry Entire Group 9 more group attempts remaining V 0 1 2 3 4 Previous Email Instructor Next Save and Exit
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 51EQ: The n-propyl cation can be formed from a molecule such as
When the C–Cl bond is broken so that...
Related questions
Question
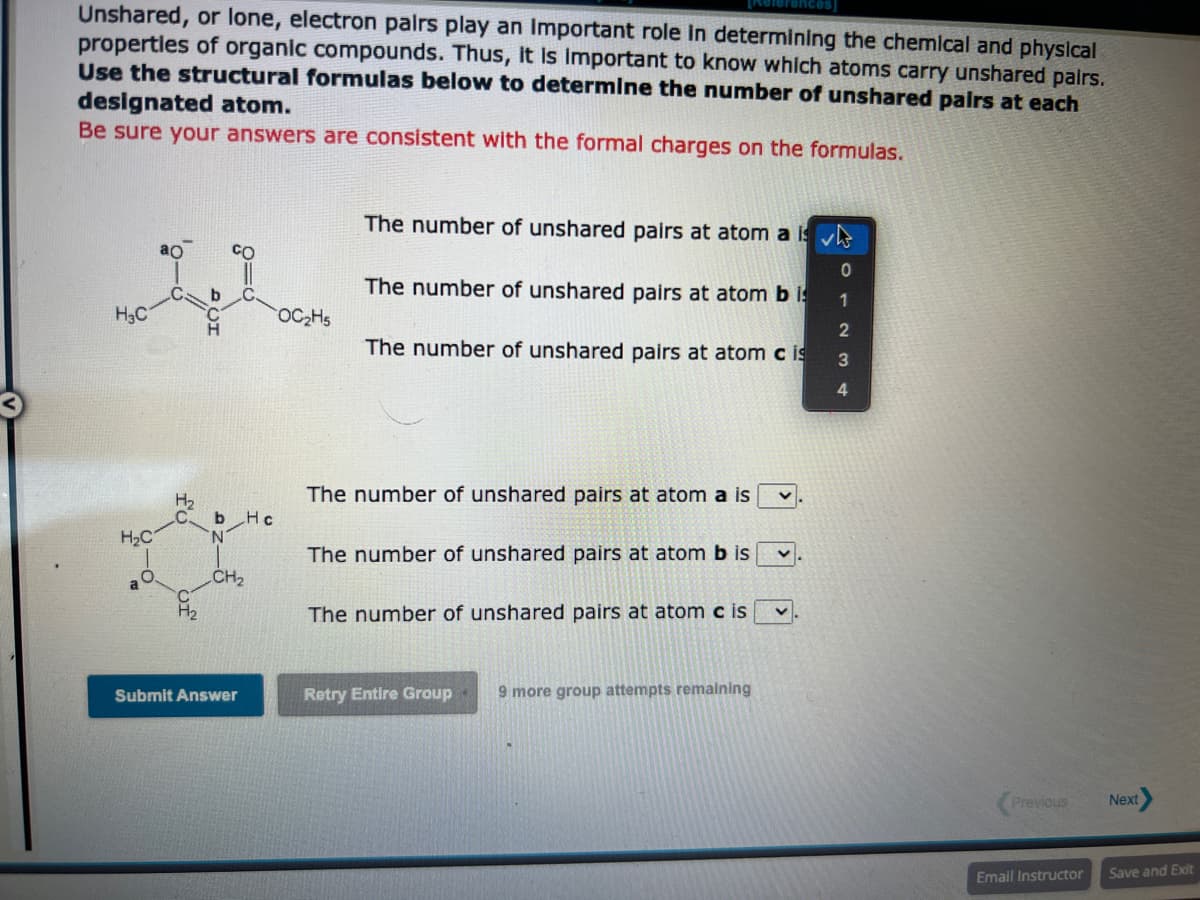
Transcribed Image Text:Unshared, or lone, electron pairs play an important role in determining the chemical and physical
properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs.
Use the structural formulas below to determine the number of unshared pairs at each
designated atom.
Be sure your answers are consistent with the formal charges on the formulas.
H3C
H₂C
a
O
ao
H₂
b
b Hc
N
CH₂
Submit Answer
OC₂H5
The number of unshared pairs at atom a is
The number of unshared pairs at atom bis
The number of unshared pairs at atom cis
The number of unshared pairs at atom a is
The number of unshared pairs at atom b is
The number of unshared pairs at atom cis
Retry Entire Group 9 more group attempts remaining
V
0
1
2
3
4
Previous
Email Instructor
Next
Save and Exit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax