Use the flow charts on the previous page to answer the following questions. In each question, a test is carried out to determine the presence or absence of several ions. Only those listed may be present. State if the tests indicate if each ion is present, absent, or undetermined. 1. Test for Ag", Cu²*, Fe³ Some 6 M HCl is added to a solution that may contain the three ions. A white precipitate forms. Ions present: Ions absent: Ions undetermined: 2. Test for Cu²*, Ag*, and Zn²* Some 6 M HCI is added to a solution that may contain the three ions. No precipitate forms. The addition of 6 M NaOH until the solution is basic results in no formation of precipitate. Ions present: Ions absent: Ions undetermined: 3. Test for Cu*, Fe*, and Zn²* 6 M NAOH is added to a clear solution that may contain the three ions until the solution is basic. A dark precipitate forms. The precipitate totally dissolves in 6 M H2SO4. The addition of 6 M NH3 to this acidic solution until it is basic results in a clear solution containing a dark precipitate. The resulting dark precipitate completely dissolves in 6 M H2SO4. Ions present: Ions absent: Ions undetermined:
Use the flow charts on the previous page to answer the following questions. In each question, a test is carried out to determine the presence or absence of several ions. Only those listed may be present. State if the tests indicate if each ion is present, absent, or undetermined. 1. Test for Ag", Cu²*, Fe³ Some 6 M HCl is added to a solution that may contain the three ions. A white precipitate forms. Ions present: Ions absent: Ions undetermined: 2. Test for Cu²*, Ag*, and Zn²* Some 6 M HCI is added to a solution that may contain the three ions. No precipitate forms. The addition of 6 M NaOH until the solution is basic results in no formation of precipitate. Ions present: Ions absent: Ions undetermined: 3. Test for Cu*, Fe*, and Zn²* 6 M NAOH is added to a clear solution that may contain the three ions until the solution is basic. A dark precipitate forms. The precipitate totally dissolves in 6 M H2SO4. The addition of 6 M NH3 to this acidic solution until it is basic results in a clear solution containing a dark precipitate. The resulting dark precipitate completely dissolves in 6 M H2SO4. Ions present: Ions absent: Ions undetermined:
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section: Chapter Questions
Problem 97GQ: A solution contains Ca2+ and Pb2+ ions, both at a concentration of 0.010 M. You wish to separate the...
Related questions
Question
Please provide explanation or solution. I am using the answer in this as an answer key to a question file I found online.
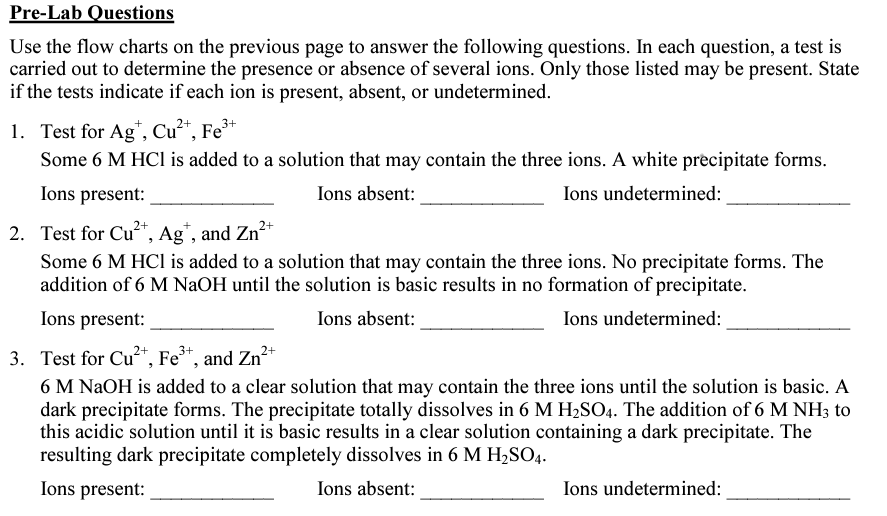
Transcribed Image Text:Pre-Lab Questions
Use the flow charts on the previous page to answer the following questions. In each question, a test is
carried out to determine the presence or absence of several ions. Only those listed may be present. State
if the tests indicate if each ion is present, absent, or undetermined.
1. Test for Ag*, Cu²“, Fe³+
Some 6 M HCI is added to a solution that may contain the three ions. A white precipitate forms.
Ions present:
Ions absent:
Ions undetermined:
2+
2. Test for Cu*, Ag", and Zn*
Some 6 M HCl is added to a solution that may contain the three ions. No precipitate forms. The
addition of 6 M NaOH until the solution is basic results in no formation of precipitate.
Ions present:
Ions absent:
Ions undetermined:
3. Test for Cu²*, Fe*, and Zn²+
6 M NAOH is added to a clear solution that may contain the three ions until the solution is basic. A
dark precipitate forms. The precipitate totally dissolves in 6 M H2SO4. The addition of 6 M NH3 to
this acidic solution until it is basic results in a clear solution containing a dark precipitate. The
resulting dark precipitate completely dissolves in 6 M H,SO4.
Ions present:
Ions absent:
Ions undetermined:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning