Use the following experimental titration data to calculate the concentration of the acid being analysed. Observations: The initial solution of acetic acid (HC2H3O2) is clear and colourless. A few drops of phenolphthalein indicator are added to each sample. A dilute solution of sodium hydroxide (concentration 2.50 x 10-4 mol/L) is used as the titrant. As the mixture reaches the endpoint, flashes of pink colour are seen and the titrant is added drop by drop. The endpoint is reached when one drop of titrant turns the mixture a pale pink colour that does not fade.
Use the following experimental titration data to calculate the concentration of the acid being analysed. Observations: The initial solution of acetic acid (HC2H3O2) is clear and colourless. A few drops of phenolphthalein indicator are added to each sample. A dilute solution of sodium hydroxide (concentration 2.50 x 10-4 mol/L) is used as the titrant. As the mixture reaches the endpoint, flashes of pink colour are seen and the titrant is added drop by drop. The endpoint is reached when one drop of titrant turns the mixture a pale pink colour that does not fade.
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 8P
Related questions
Question
Use the following experimental titration data to calculate the concentration of the acid being analysed.
Observations: The initial solution of acetic acid (HC2H3O2) is clear and colourless. A few drops of phenolphthalein indicator are added to each sample. A dilute solution of sodium hydroxide (concentration 2.50 x 10-4 mol/L) is used as the titrant. As the mixture reaches the endpoint, flashes of pink colour are seen and the titrant is added drop by drop. The endpoint is reached when one drop of titrant turns the mixture a pale pink colour that does not fade.
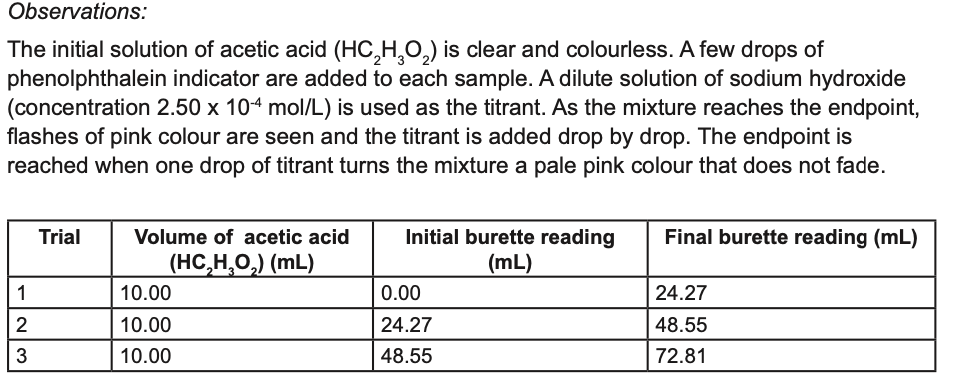
Transcribed Image Text:Observations:
The initial solution of acetic acid (HC,H,0,) is clear and colourless. A few drops of
phenolphthalein indicator are added to each sample. A dilute solution of sodium hydroxide
(concentration 2.50 x 104 mol/L) is used as the titrant. As the mixture reaches the endpoint,
flashes of pink colour are seen and the titrant is added drop by drop. The endpoint is
reached when one drop of titrant turns the mixture a pale pink colour that does not fade.
Trial
Volume of acetic acid
Initial burette reading
Final burette reading (mL)
(HC,H,O,) (mL)
(mL)
1
10.00
0.00
24.27
2
10.00
24.27
48.55
3
10.00
48.55
72.81
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

