Use the ICE table method to calculate the pH of a 0.89 M NH3 solution. Kp = 1.8 x 10-5 %3D IMPORTANT: When entering your answer: • Enter the number only (no "pH =" even though it feels so wrong) • Do not leave any spaces • Use a leading zero before the decimal when necessary • Report your number to 2 decimal places (regardless of the significant figures) Correctly round your answer to the 2nd decimal place (allowed margin of error is only ± 0.01, so always use un- rounded numbers in your calculations) Examples: -0.12 or 12.34
Use the ICE table method to calculate the pH of a 0.89 M NH3 solution. Kp = 1.8 x 10-5 %3D IMPORTANT: When entering your answer: • Enter the number only (no "pH =" even though it feels so wrong) • Do not leave any spaces • Use a leading zero before the decimal when necessary • Report your number to 2 decimal places (regardless of the significant figures) Correctly round your answer to the 2nd decimal place (allowed margin of error is only ± 0.01, so always use un- rounded numbers in your calculations) Examples: -0.12 or 12.34
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 7ALQ
Related questions
Question
please show work
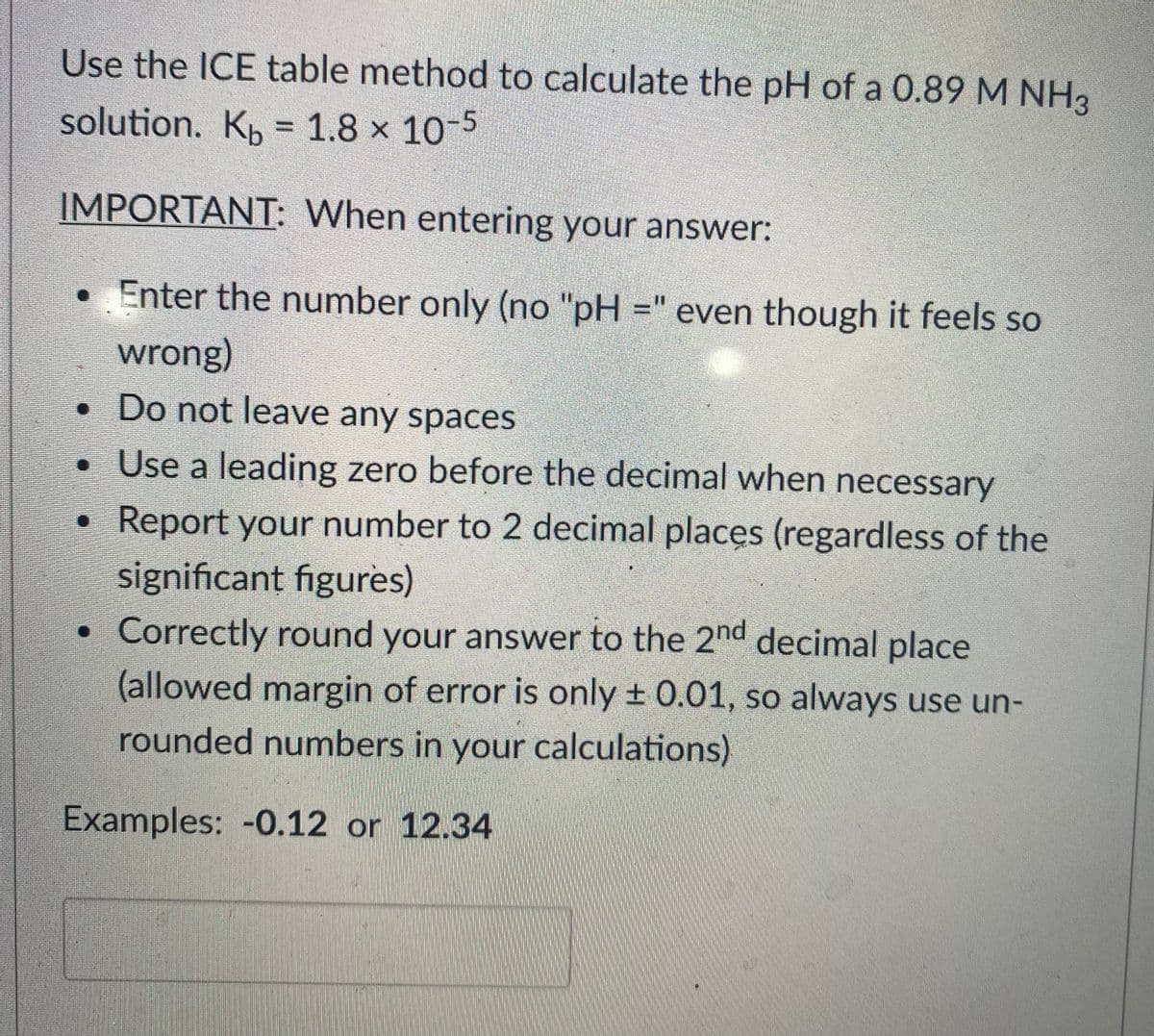
Transcribed Image Text:Use the ICE table method to calculate the pH of a 0.89 M NH3
solution. K, = 1.8 × 10-5
IMPORTANT: When entering your answer:
• Enter the number only (no "pH =" even though it feels so
wrong)
• Do not leave any spaces
• Use a leading zero before the decimal when necessary
Report your number to 2 decimal places (regardless of the
significant figures)
Correctly round your answer to the 2nd decimal place
(allowed margin of error is only + 0.01, so always use un-
rounded numbers in your calculations)
Examples: -0.12 or 12.34
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning