Q: The phase diagram of substance W is shown. a. Suggest one application of the phase diagram of W. b.…
A: Phase diagram: It is a representation of the physical state of a substance in different conditions…
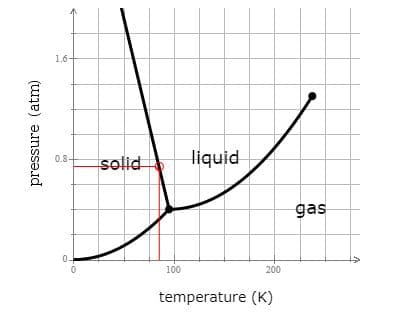
Q: In the phase diagram shown, segment AD corresponds to the conditions of temperature and pressure…
A: The phase diagram given is,
Q: Which of the following statements is wrong The temperature below which the liquid state of a…
A: The wrong statements can be choosen as follows:
Q: Recognize the features of molecules that favor formation of liquid crystalline phases?
A: Liquid crystal are the substances which have mixture of properties of both liquid and crystal.
Q: The vapor pressure of liquid octane, C3H15, is 100. mm Hg at 339 K. A sample of CgH1g is placed in a…
A: From ideal gas equation, PV=nRT Here, P is pressure, V is volume, n is the number of moles, R is the…
Q: Could you measure the triple point of water by measuring the temperature in a vessel in which water…
A: Phase diagram represents the physical states of substance at different pressure and temperature in a…
Q: What is the boiling point of water in a pressure cooker with a pressure of 2.00 atm? (The enthalpy…
A: Using clausius - clapeyron equation for finding boiling point of water in pressure cooker The…
Q: For each pair, predict which one will have the higher value for vapor pressure when it is in the…
A: For each pair, predict which one will have the higher value for vapor pressure when it is in the…
Q: (b) Why does the liquid-gas coexistence curve in a P-T phase diagram end at the critical point? Draw…
A: The three different phases that are common for a substance are solid, liquid, and gas. In the solid…
Q: A sample is initially at the temperature and pressure indicated by point (C) on the phase diagram…
A:
Q: 12. In the accompanying phase diagram, a liquid can be present at combinations of temperature and…
A: A phase is a physically distinct boundary which is separable. In a phase diagram of a compound, as…
Q: Predict the phase of water that exists under the following conditions: a) P = 200 kPa, T=110°C b) P…
A: Phase diagram of water:
Q: Based on the concept of phase diagrams, what information on the property of liquids can you get from…
A: Phase diagram is a graphical representation of the physical states of a substance under various…
Q: Given that the pressure in a phase diagram remains constant, what type/s of phase changes can occur…
A:
Q: The phase diagram for carbon is shown.
A: Triple point is considered to be tgat point where 3 phases exist together in the phase diagram
Q: What is the vapor pressure of a liquid (in mmHg) at 299.17 K if its AHvap = 28.9 kJ/mol and its…
A:
Q: The vapor pressure, P, of a certain liquid was measured at two temperatures, T. The data is shown in…
A: Plot of ln(P) Vs 1/T, to determine the enthalpy of vaporisation.
Q: The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol , and its normal boiling…
A: Given : Molar enthalpy of vaporization of CS2 = 26.74 kJ/MolNormal boiling point = 46°cTemperature =…
Q: Which of the following phase diagrams corresponds to a system wherein boiling occurs before the two…
A: For the liquid-liquid phase diagram, the phase diagram that represents a system wherein boiling…
Q: 6.The diagram below is an example of Liquid-Vapor(LV) Pressure (P vs Mole Fraction).Fill out the…
A: The defined behaviour of liquid and vapour of a molecule can be well studied by considering two…
Q: The molar enthalpy of vaporization of hexane (C6H14) is 28.9 kJ/mol, and its normal boiling point is…
A: Clausius – Clapeyron equation is used to calculate the vapor pressure at another temperature…
Q: Consider the following phase diagram: Pressure (atm) 1.0 0.5 0 O 50 K O 100 K 200 K D O 300 K O 360…
A: 0° C = 273 K.... 1 atm = 760 torr... The boiling point is the temperature at which a liquid changes…
Q: The next phase diagram looks at the effect of changing pressure at constant temperature. Name and…
A: Here phase diagram given I have explained below with details about critical point.
Q: Use the phase diagram of Substance X below to find the melting point of X when the pressure above…
A: Phase diagram is a graphical representation of the physical states of a substance under different…
Q: What is the Critical point and Triple point of the phase diagram?
A: Given: Phase diagram To find: Critical point and triple point Solution: Phase diagram is the graph…
Q: Visualizing physical states in a phase diagram Match each numbered point (1-3) in the phase diagram…
A:
Q: Which of the following graphs best represents the change in the total energy vs. temperature as N₂O…
A: In the given phase diagram of N2O, The far left region shows the solid phase of N2O. The far right…
Q: At the boiling point of carbon disulfide, CS2 , ∆Hvap = 26.9 kJ/mol and ∆Svap = 84.5 J/mol.K.…
A: The boiling point can be calculated as follows-
Q: The large circle in the center of the phase diagram represents which point? В 1 A Temperature (°C) o…
A: Given diagram,
Q: Refer to Fig. 4A.9. Which phase or phases would you expect to be present for a sample of H2O at: (i)…
A: 1Pa = 9.869 x 10-6 atm so 1 atm = 101325 Pa (i) So at 100 k,1 atm a sample of H2O would be in the…
Q: At which point in the phase diagram below are solid and liquid phases in equilibrium? Phase Diagram…
A: Find out which point solid and liquid phase at eualibrium.
Q: For the process X(1) X(g), where X is a metal, AH°-433.4 kJ/mol and AS°=101.1 J/(K mol). What is the…
A: GivenX(l) → X (g)∆H° = 433.4 kJ/mol1 kJ = 1000 J∆H° = 433400 J/mol∆S° = 101.1 J/K.mol
Q: Which line must the temperature and pressure have crossed if a iquid sample of X is observed to…
A: 1) X is a gas. X is observed to freeze at point F where temperature and pressure have crossed. 2) In…
Q: Rank the substances from in order of expected melting point, based on the relative strengths of the…
A: If the molecules have stronger force of attraction between their atoms then the requirement of force…
Q: pressure 4 x x 10 3 x k 5 x 6 2 x 8 x 9 temperature
A: In the given phase diagram, T represents the triple point and C represents the critical point Other…
Q: phase diagram for xenon : 57.6 1.00 P atm 0.37 152.0 161.3 165.0 289.7 What is the normal freezing…
A: The temperature of the substance on which the liquid is converted into the solid-state is known as…
Q: From the plot of vapor pressures vs temperature above, estimate the boiling point of carbon…
A: Boiling point of a liquid is defined as the temperature at which the vapor pressure of the liquid…
Q: What is the phase change from 82 to 86 K at a constant 1.0 atm? *
A: The phase diagram represents the changes in the phase of a compound upon variation of temperature…
Q: The temperature dependence of the vapor pressure of solid sulfur dioxide can be approximated by…
A: Given that, Temperature dependence of vapor pressure of solid SO2 is = logp = 10.5916 - 1871.2TK…
Q: What is the significance of the triple point in a phase diagram?
A: All matter can be classified as solid, liquid and gases mainly. In these physical states of matter,…
Q: 900 800 Carbon disulfide 700 600 Methanol 500 Ethanol 400 Heptane 300 200 100 10 20 30 40 50 60 70…
A: 1- Here vapour pressure for heptane is given 435mmHg first you take a middle point between 420mmHg…
Q: What is the vapor pressure of a liquid (in mmHg) at 305.13 K if its AHvap = 28.9 kJ/mol and its…
A: Physical chemistry.
Q: The molar enthalpy of hexane (C6H14) is 28.9 kJ/mol, and its normal boiling point is 68.73 °C. What…
A: The normal boiling point is the temperature when external pressure equals 760 mmHg.Clausius –…
Q: Describe the major features of the phase diagram?
A: The major features of a phase diagram is, They are phase boundaries and they have triple point. This…
Q: pressure 4 x x 10 3 x x 6 T. x 8 1 x temperature 2.
A: Answer: Phase diagram of a substance give us the interpretation of different physical states of that…
Q: 7. The molar enthalpy of vaporization of hexane (C6H14) is 28.9 kJ/mol, and its normal boiling point…
A:
Q: The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its norml boiling point…
A: Clausius-Clapeyron equation pertains to the relationship between the pressure and temperature. It is…
Q: What is the vapor pressure of a liquid (in mmHg) at 317.21 K if its AHvap = 28.9 kJ/mol and its…
A: The solution is as follows :
Q: Dichloromethane (CH2Cl2, Hvap= 25 kj/mol) has a vapor pressure of 330 mm Hg at 20oC. What is the…
A: Given,ΔH = 25 kj/molP2 = 330 mm Hg
Q: The density of solid gallium at its melting point is 5.9 g/cm³, whereas that of liquid gallium is…
A: Whether the temperature of liquid gallium at the triple point is higher or lower than normal boiling…
Use the phase diagram of Substance X below to find the pressure at which the melting point of X is −181.°C
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Pressure (P) Volume (V) Temperature (T) Moles (n) 1. 5.00 atm 25.00 L 273.15 K 2. 0.55 L 308 K 0.50 mol 3. 20.00 atm 30.00 L 25.30 mol 4. 15.00 atm 370.00 K 3.00 mol 5. 10.50 L 280.00 K 10.00 mol5.Measures to observe when measuring viscous liquids include the following, with exception ofa.Use of silicone treated glassware for smaller volume of liquidsb.Pouring from bottle that is not completely filledc.When pouring, do not allow the liquid to run down the inside surface of the graduatesd.Drainage time is longer6.Most accurate balance to use for pharmaceutical analysisa.Household scaleb.Top loading balancec.Analytical balanceTriple beam balanceThe sublimation point of CO2 (solid) at 1 atm is -78.37oC and the enthalpy of sublimation(solid to vapor) is 3116 joule/mole. cp (CO2(solid))=50 joule/mole.K. and for cp CO2(g) lookat Appendix.5 gram CO2 gasis held at T=300/X oK in container for some times as represented below:CO2 (gas) at ToK ==➔Dry Ice (Solid CO2) at ToKCalculate:a) The enthalpy of deposition at ToKb) The entropy of deposition at ToK.c) The entropy change of surroundings at ToK. Assume surroundings behavereversibly. Surrounding temperature is ToKd) Entropy change and entropy produced of universe.e) Is this deposition process spontaneous? prove! At given temperature T. x=4.5
- Virial equation can be written in Z (compressibility factor form): Z = pV/RT = 1 + B'p + C'p2 + D'p3 + ... Z = 1 + (B/V) + (C/V2) + (D/V3) + ... Show that C'=(C-B2)/(RT)2 on each Z (compressibility factor form) above.A parcel of unsaturated air contains a mixture of dry air and water vapor. The specific humidity q of the air parcel is 12 g kg^−1. Recall that q = Mv / (Mv+Md),where Mv is the mass of the water vapor and Md is the mass of the dry air. This is equivalent to what we have been calling the fractional concentration of water vapor by mass. (a) If the air parcel is at a temperature T= 25◦C , what is the virtual temperature of the air parcel? (b) If the air parcel is at a temperature T= 25◦C and a pressure of 1000 hPa, what is the relative humidity of the air parcel? (c) What is the apparent molar mass of the air parcel?I need help understanding. I know thag is not 2 nor 3. Will it be 4 because there is 4gas phase
- 4. A sphere of radius 5 x10-2 cm and density of 1.1 g/cc falls at constant velocity through a liquid of density 1.0 g/cc and viscosity of 1 poise. What is the velocity of the falling sphere?An open tank is filled with a liquid that has a specific gravity of 1 .45. The liquid exerts a head pressure of 355 inches w.c. What is the level in inches?P1A.6 The molar mass of a newly synthesized fluorocarbon was measured in a gas microbalance. is device consists of a glass bulb forming one end of a beam, the whole surrounded by a closed container. e beam is pivoted, and the balance point is attained by raising the pressure of gas in the container, so increasing the buoyancy of the enclosed bulb. In one experiment, the balance point was reached when the fluorocarbon pressure was 327.10Torr; for the same setting of the pivot, a balance was reached when CHF3 (M = 70.014 g mol−1) was introduced at 423.22 Torr. A repeat of the experiment with a di erent setting of the pivot required a pressure of 293.22 Torr of the uorocarbon and 427.22 Torr of the CHF3. What is the molar mass of the fluorocarbon? Suggest a molecular formula.
- 1. Estimate the density of a 25-API gravity dead oil at 100 F.Determine the viscosity of diethyl ether at 77 degrees Celsius. (Pa·s) Determine the density of cyclohexene at 50 degrees Celsius. (kg/m3)Show complete solutions and enclose all final answers in a box. Round off final answers to 4 decimal places and use floating values for intermediate answers. A gas column is separated into two segments by a non-permeable partition. On the first segment is 1.63 moles of Helium with volume V1 and on the second segment is 3.84 moles of Oxygen gas with volume V2. Consider both gases to be ideal and at the same pressure P and temperature T. If the partition is removed, calculate the change in entropy of the gaseous system.

