Use the References to access important values if needed for A sample of chlorine contains 24.3 grams. 1. How many moles of Cl atoms are there in the sample? mol Cl 2. How many moles of Cl, molecules are there in the sample? mol Cl2
Use the References to access important values if needed for A sample of chlorine contains 24.3 grams. 1. How many moles of Cl atoms are there in the sample? mol Cl 2. How many moles of Cl, molecules are there in the sample? mol Cl2
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 100AP: f you have equal mole samples of NO2 and F2 , which of the following must be true? l type='a'> The...
Related questions
Question
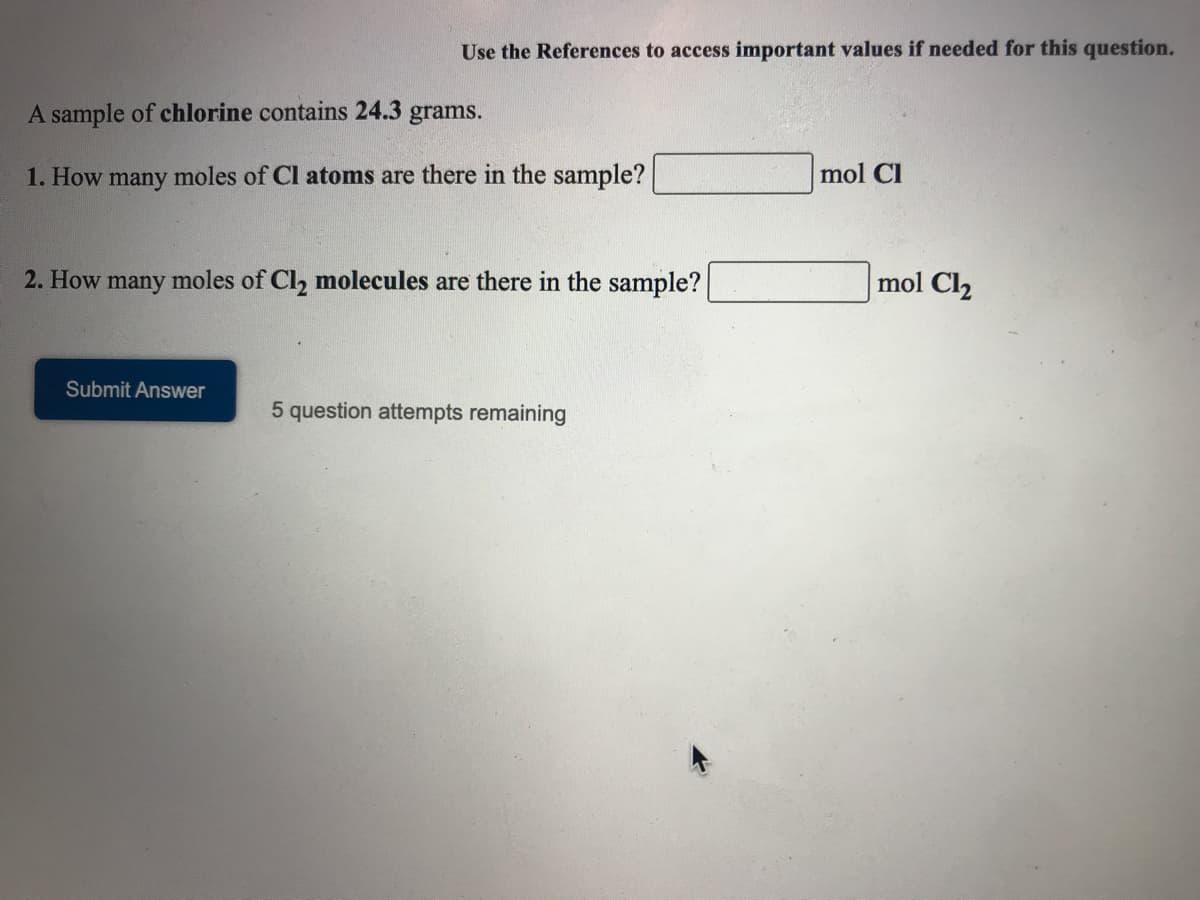
Transcribed Image Text:Use the References to access important values if needed for this question.
A sample of chlorine contains 24.3 grams.
1. How many moles of Cl atoms are there in the sample?
mol Cl
2. How many moles of Cl, molecules are there in the sample?
mol Cl2
Submit Answer
5 question attempts remaining
Expert Solution
Step 1
Elemental form of chlorine is Chlorine gas i.e. Cl2
A mole of Cl2 contains two moles of Cl.
Atomic mass of chlorine is 35.5g/mol
Atomic mass of Cl2 will be 71g/mol
No. of moles of Cl2 = given mass/atomic or molar mass.
=24.3/71
=.342 moles
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax