Use the References to access important values if needed for this question. a. Use strain energy increments in the OWL Table Reference (sce References button, Strain Energy Increments) to calculate the energy difference between the two chair conformations of the compound below. b. Specify substituent positions (axial or equatorial) in the more stable chair. c. Estimate the percent of the more stable chair at equilibrium at 25°C. (To determine the percent of the more stable chair at equilibrium, first calculate K, and then use this value to find the percentage.) CH3 CI Answers: a. The energy difference is b. In the more stable chair: kJ/mol. • The chloro group is in the Oposition. • The methyl group is in the position. c. At 25°C the equilibrium percent of the more stable chair conformation is approximately
Use the References to access important values if needed for this question. a. Use strain energy increments in the OWL Table Reference (sce References button, Strain Energy Increments) to calculate the energy difference between the two chair conformations of the compound below. b. Specify substituent positions (axial or equatorial) in the more stable chair. c. Estimate the percent of the more stable chair at equilibrium at 25°C. (To determine the percent of the more stable chair at equilibrium, first calculate K, and then use this value to find the percentage.) CH3 CI Answers: a. The energy difference is b. In the more stable chair: kJ/mol. • The chloro group is in the Oposition. • The methyl group is in the position. c. At 25°C the equilibrium percent of the more stable chair conformation is approximately
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 2E
Related questions
Question
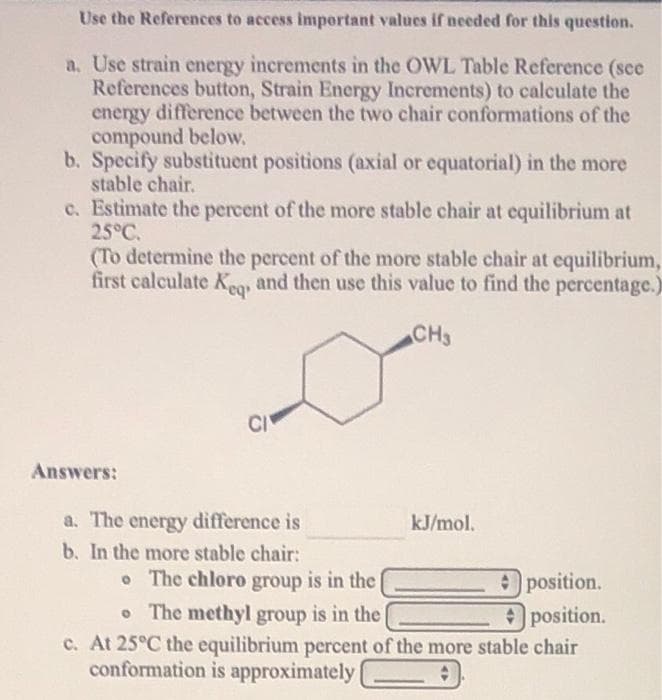
Transcribed Image Text:Use the References to access important values if needed for this question.
a. Use strain energy increments in the OWL Table Reference (sce
References button, Strain Energy Increments) to calculate the
energy difference between the two chair conformations of the
compound below.
b. Specify substituent positions (axial or equatorial) in the more
stable chair.
c. Estimate the percent of the more stable chair at equilibrium at
25°C.
(To determine the percent of the more stable chair at equilibrium,
first calculate K, and then use this value to find the percentage.)
CH3
CI
Answers:
a. The energy difference is
b. In the more stable chair:
kJ/mol.
o The chloro group is in the
position.
• The methyl group is in the
Oposition.
c. At 25°C the equilibrium percent of the more stable chair
conformation is approximately
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning