Use the slope of the line to find the absorptivity of 2+ Cu(NH3)4 at 600 nm. 2+ Use the absorptivity from question 4 to find the molar concentration of Cu(NH3)42* in each of the unknown test solutions.
Use the slope of the line to find the absorptivity of 2+ Cu(NH3)4 at 600 nm. 2+ Use the absorptivity from question 4 to find the molar concentration of Cu(NH3)42* in each of the unknown test solutions.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 5ALQ: Can the subscripts in a chemical formula be fractions? Explain.
Related questions
Question
Please help with questions 4, 5, 6 and 7. The equation of the line is y = 0.0966x+0.205
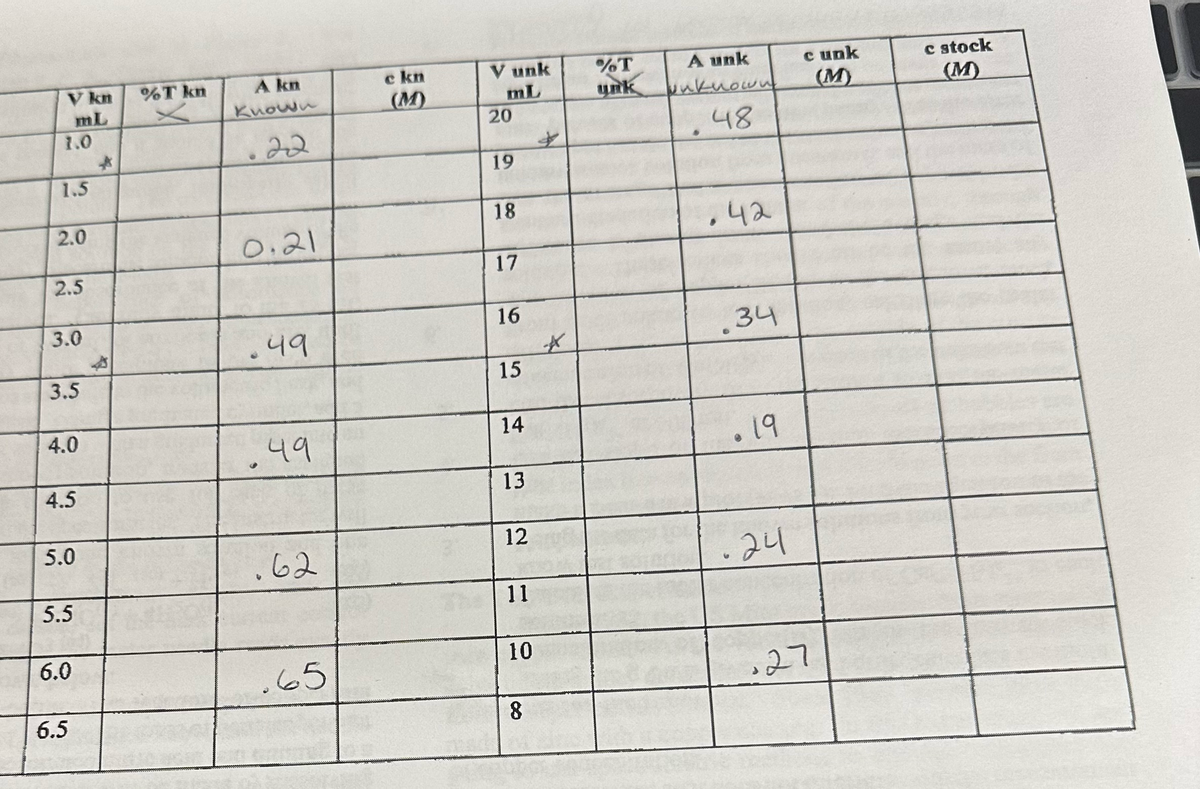
Transcribed Image Text:V kn
mL
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
5.5
6.0
6.5
*
%T kn
A kn
Known
.22
0.21
.49
49
.62
.65
c kn
V unk
mL
20
19
18
17
16
15
14
13
12
11
10
8
*
%T
unk
A unk
unknown
48
.42
.34
• 19
24
cunk
(M)
27
c stock
(M)
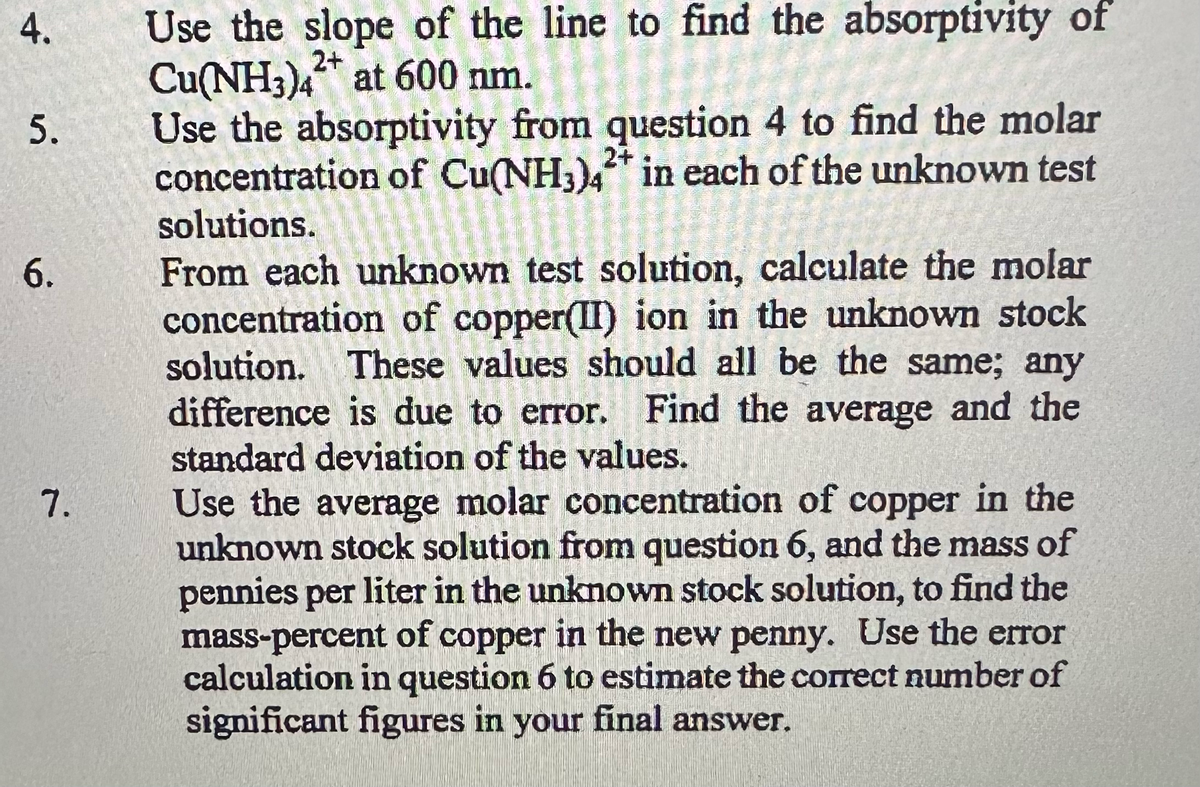
Transcribed Image Text:4.
5.
6.
7.
Use the slope of the line to find the absorptivity of
Cu(NH3)4 at 600 nm.
2+
Use the absorptivity from question 4 to find the molar
concentration of Cu(NH3)4* in each of the unknown test
solutions.
From each unknown test solution, calculate the molar
concentration of copper(II) ion in the unknown stock
solution. These values should all be the same; any
difference is due to error. Find the average and the
standard deviation of the values.
Use the average molar concentration of copper in the
unknown stock solution from question 6, and the mass of
pennies per liter in the unknown stock solution, to find the
mass-percent of copper in the new penny. Use the error
calculation in question 6 to estimate the correct number of
significant figures in your final answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning
