Use the standard reaction enthalpies given below to determine AH°rxn for the following reaction: P4(g) + 10 Cl2(g) - 4 PCI5(s) AH°rxn = ??? Given: PCI5(s) → PCI3(g) + Cl2(g) AH°rxn = +157 kJ %3D P4(g) + 6 Cl,(g) → 4 PCI3(g) AH°rxn = -1207 kJ -519 kJ -2115 kJ -1366 kJ -1835 kJ -1055 kJ
Use the standard reaction enthalpies given below to determine AH°rxn for the following reaction: P4(g) + 10 Cl2(g) - 4 PCI5(s) AH°rxn = ??? Given: PCI5(s) → PCI3(g) + Cl2(g) AH°rxn = +157 kJ %3D P4(g) + 6 Cl,(g) → 4 PCI3(g) AH°rxn = -1207 kJ -519 kJ -2115 kJ -1366 kJ -1835 kJ -1055 kJ
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 67E: The enthalpy of combustion of solid carbon to form carbon dioxide is 393.7 KJ/mol carbon, and the...
Related questions
Question
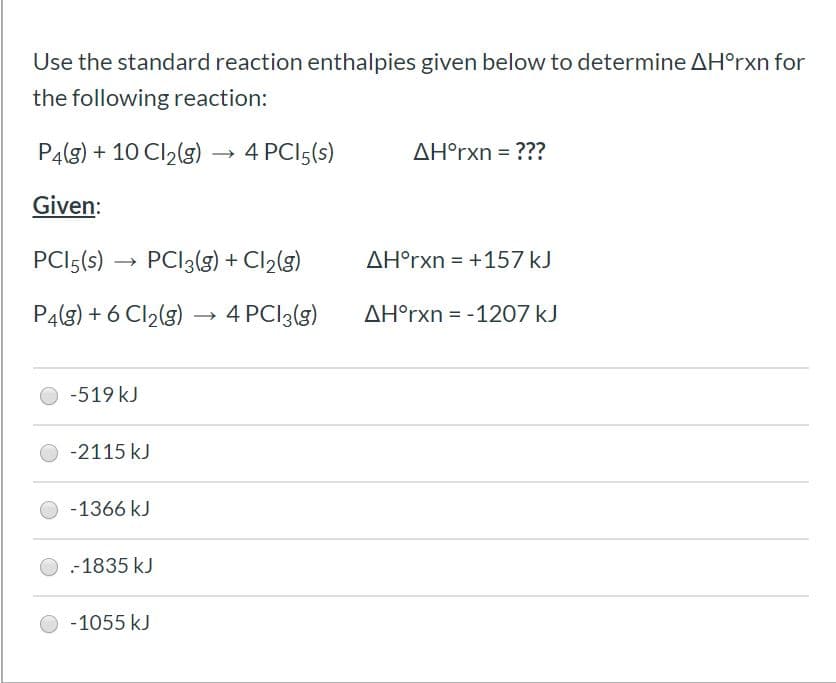
Transcribed Image Text:Use the standard reaction enthalpies given below to determine AH°rxn for
the following reaction:
P4(g) + 10 Cl2(g) -
4 PCI5(s)
AH°rxn = ???
Given:
PCI5(s) →
PCI3(g) + Cl2(g)
AH°rxn = +157 kJ
%3D
P4(g) + 6 Cl,(g) → 4 PCI3(g)
AH°rxn = -1207 kJ
-519 kJ
-2115 kJ
-1366 kJ
-1835 kJ
-1055 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning