Use the standard reduction potentials located in the 'Tables' linked above to calculate the standard free energy change in kJ for the reaction: Hg2*(aq) + Co(s)→ Hg(l) + Co²*(aq) Answer: kJ K for this reaction would b than one. greater less Standard Reduction (Electrode) Potentials at 25 °C Half-Cell Reaction E° (volts) F2(g) + 2 e→2 F(aq) 2.87 Ce4*(aq) + e –→ Ce"(aq) 3+ 1.61 2+ MnO4 (aq) + 8 H"(aq) + 5 e¯ –→ Mn-T(aq) + 4 H2O(1) 1.51 Cl2(g) + 2 e' —2 СГ (aq) 1.36 Cr2072"(aq) + 14 H*(aq) + 6 e¯ →2 Cr"(aq) + 7 H2O(1) 1.33 O2(g) + 4 H*(aq) + 4 e→2 H2O(1) 1.229 Br2(1) + 2 e¯ –→2 Br¯(aq) 1.08 NO3 (aq) + 4 H"(aq) +3 e¯ –→ NO(g) + 2 H2O(1) 0.96 2 Hg-*(aq) + 2 e¯ → Hg22 (aq) 0.920 Hg-"(aq) + 2 e →Hg(1) 0.855 Ag"(aq) + e¯ – Ag(s) 0.799 2+, Hg2"(aq) + 2 e¯ →2 Hg(1) 0.789 Fe*(aq) + e → Fe2*(aq) 0.771 I2(s) + 2 e → 2 1 (aq) 0.535 Fe(CN)6° (aq) + e→ Fe(CN)64 (aq) 0.48 Cu2*(aq) + 2 e → Cu(s) 0.337 Cu2*(aq) + e" – Cu"(aq) 0.153 S(s) + 2 H*(aq) + 2 e → H2S(aq) 0.14 2 H*(aq) + 2 e → H2(g) 0.0000 Pb2*(aq) + 2 e → Pb(s) -0.126 Sn-"(aq) + 2 e¯ → Sn(s) „2+ -0.14 Ni2+(ag) + 2 e →Ni(s) -0.25 Co2+(ag) + 2 e →Co(s) -0.28 Cd2*(aq) + 2 e →Cd(s) -0.403 Cr3+ C* (aq) (aq) + e -0.41 Fe2*(aq) + 2 e →Fe(s) -0.44 Cr3+ (aq) + 3 e → Cr(s) -0.74 Zn2*(aq) + 2 e →Zn(s) -0.763 2 H20(1) + 2 e → H2(g) + 2 OH (aq) -0.83 Mn-"(aq) + 2 e¯ → Mn(s) 2+ -1.18 Als*(aq) + 3 e → Al(s) -1.66 2+ Mg"(aq) + 2 e →Mg(s) -2.37 Na"(aq) + e –→ Na(s) -2.714 K(aq) + e→ K(s) -2.925 Li*(aq) + e Li(s) -3.045
Use the standard reduction potentials located in the 'Tables' linked above to calculate the standard free energy change in kJ for the reaction: Hg2*(aq) + Co(s)→ Hg(l) + Co²*(aq) Answer: kJ K for this reaction would b than one. greater less Standard Reduction (Electrode) Potentials at 25 °C Half-Cell Reaction E° (volts) F2(g) + 2 e→2 F(aq) 2.87 Ce4*(aq) + e –→ Ce"(aq) 3+ 1.61 2+ MnO4 (aq) + 8 H"(aq) + 5 e¯ –→ Mn-T(aq) + 4 H2O(1) 1.51 Cl2(g) + 2 e' —2 СГ (aq) 1.36 Cr2072"(aq) + 14 H*(aq) + 6 e¯ →2 Cr"(aq) + 7 H2O(1) 1.33 O2(g) + 4 H*(aq) + 4 e→2 H2O(1) 1.229 Br2(1) + 2 e¯ –→2 Br¯(aq) 1.08 NO3 (aq) + 4 H"(aq) +3 e¯ –→ NO(g) + 2 H2O(1) 0.96 2 Hg-*(aq) + 2 e¯ → Hg22 (aq) 0.920 Hg-"(aq) + 2 e →Hg(1) 0.855 Ag"(aq) + e¯ – Ag(s) 0.799 2+, Hg2"(aq) + 2 e¯ →2 Hg(1) 0.789 Fe*(aq) + e → Fe2*(aq) 0.771 I2(s) + 2 e → 2 1 (aq) 0.535 Fe(CN)6° (aq) + e→ Fe(CN)64 (aq) 0.48 Cu2*(aq) + 2 e → Cu(s) 0.337 Cu2*(aq) + e" – Cu"(aq) 0.153 S(s) + 2 H*(aq) + 2 e → H2S(aq) 0.14 2 H*(aq) + 2 e → H2(g) 0.0000 Pb2*(aq) + 2 e → Pb(s) -0.126 Sn-"(aq) + 2 e¯ → Sn(s) „2+ -0.14 Ni2+(ag) + 2 e →Ni(s) -0.25 Co2+(ag) + 2 e →Co(s) -0.28 Cd2*(aq) + 2 e →Cd(s) -0.403 Cr3+ C* (aq) (aq) + e -0.41 Fe2*(aq) + 2 e →Fe(s) -0.44 Cr3+ (aq) + 3 e → Cr(s) -0.74 Zn2*(aq) + 2 e →Zn(s) -0.763 2 H20(1) + 2 e → H2(g) + 2 OH (aq) -0.83 Mn-"(aq) + 2 e¯ → Mn(s) 2+ -1.18 Als*(aq) + 3 e → Al(s) -1.66 2+ Mg"(aq) + 2 e →Mg(s) -2.37 Na"(aq) + e –→ Na(s) -2.714 K(aq) + e→ K(s) -2.925 Li*(aq) + e Li(s) -3.045
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section17.6: E⁰cell, Gibbs Free Energy, And K⁰
Problem 17.6PSP
Related questions
Question
100%
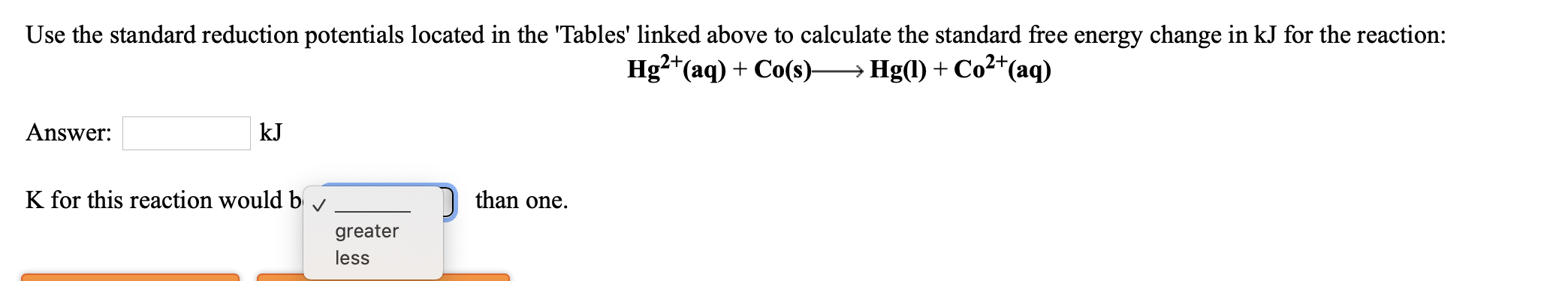
Transcribed Image Text:Use the standard reduction potentials located in the 'Tables' linked above to calculate the standard free energy change in kJ for the reaction:
Hg2*(aq) + Co(s)→ Hg(l) + Co²*(aq)
Answer:
kJ
K for this reaction would b
than one.
greater
less
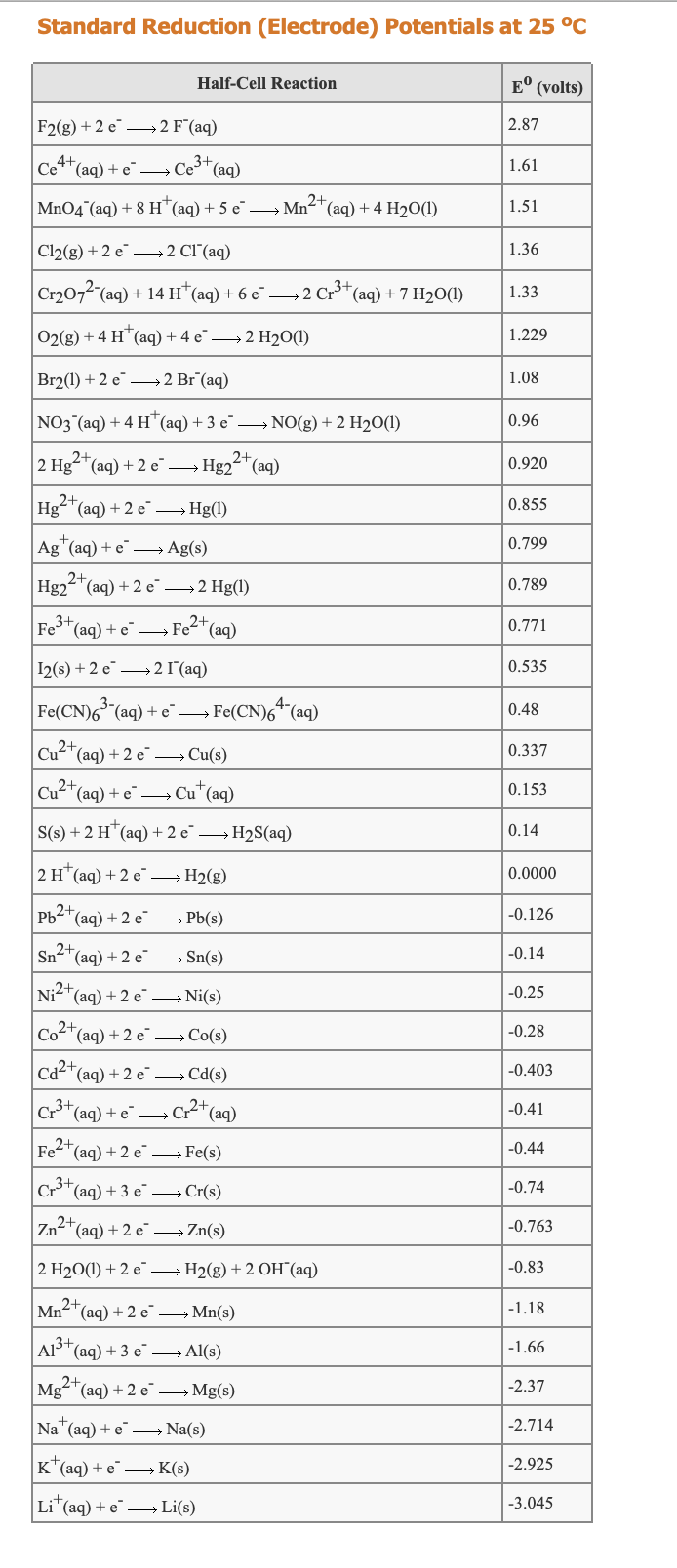
Transcribed Image Text:Standard Reduction (Electrode) Potentials at 25 °C
Half-Cell Reaction
E° (volts)
F2(g) + 2 e→2 F(aq)
2.87
Ce4*(aq) + e –→ Ce"(aq)
3+
1.61
2+
MnO4 (aq) + 8 H"(aq) + 5 e¯ –→ Mn-T(aq) + 4 H2O(1)
1.51
Cl2(g) + 2 e' —2 СГ (aq)
1.36
Cr2072"(aq) + 14 H*(aq) + 6 e¯
→2 Cr"(aq) + 7 H2O(1)
1.33
O2(g) + 4 H*(aq) + 4 e→2 H2O(1)
1.229
Br2(1) + 2 e¯ –→2 Br¯(aq)
1.08
NO3 (aq) + 4 H"(aq) +3 e¯ –→ NO(g) + 2 H2O(1)
0.96
2 Hg-*(aq) + 2 e¯ →
Hg22 (aq)
0.920
Hg-"(aq) + 2 e →Hg(1)
0.855
Ag"(aq) + e¯ – Ag(s)
0.799
2+,
Hg2"(aq) + 2 e¯ →2 Hg(1)
0.789
Fe*(aq) + e →
Fe2*(aq)
0.771
I2(s) + 2 e → 2 1 (aq)
0.535
Fe(CN)6° (aq) + e→ Fe(CN)64 (aq)
0.48
Cu2*(aq) + 2 e → Cu(s)
0.337
Cu2*(aq) + e" – Cu"(aq)
0.153
S(s) + 2 H*(aq) + 2 e → H2S(aq)
0.14
2 H*(aq) + 2 e → H2(g)
0.0000
Pb2*(aq) + 2 e → Pb(s)
-0.126
Sn-"(aq) + 2 e¯ → Sn(s)
„2+
-0.14
Ni2+(ag) + 2 e →Ni(s)
-0.25
Co2+(ag) + 2 e →Co(s)
-0.28
Cd2*(aq) + 2 e →Cd(s)
-0.403
Cr3+
C* (aq)
(aq) + e
-0.41
Fe2*(aq) + 2 e →Fe(s)
-0.44
Cr3+
(aq) + 3 e → Cr(s)
-0.74
Zn2*(aq) + 2 e →Zn(s)
-0.763
2 H20(1) + 2 e → H2(g) + 2 OH (aq)
-0.83
Mn-"(aq) + 2 e¯ → Mn(s)
2+
-1.18
Als*(aq) + 3 e → Al(s)
-1.66
2+
Mg"(aq) + 2 e →Mg(s)
-2.37
Na"(aq) + e –→ Na(s)
-2.714
K(aq) + e→ K(s)
-2.925
Li*(aq) + e Li(s)
-3.045
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning