Using curved arrows to show movement of electron pairs, show the mechanism for this reaction. It is only necessary to draw one resonance structure for any intermediates that are resonance hybrids. Note that the H atom of the hydroxyl group is acidic enough to be removed by OH–; the first step in the mechanism is a proton transfer. The general mechanism for base-mediated saponification of an ester is outlined in one of the discussion videos that has been posted for this experiment.
Using curved arrows to show movement of electron pairs, show the mechanism for this reaction. It is only necessary to draw one resonance structure for any intermediates that are resonance hybrids. Note that the H atom of the hydroxyl group is acidic enough to be removed by OH–; the first step in the mechanism is a proton transfer. The general mechanism for base-mediated saponification of an ester is outlined in one of the discussion videos that has been posted for this experiment.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 75P
Related questions
Question
Using curved arrows to show movement of electron pairs, show the mechanism for this reaction. It is only necessary to draw one resonance structure for any intermediates that are resonance hybrids. Note that the H atom of the hydroxyl group is acidic enough to be removed by OH–; the first step in the mechanism is a proton transfer. The general mechanism for base-mediated saponification of an ester is outlined in one of the discussion videos that has been posted for this experiment.
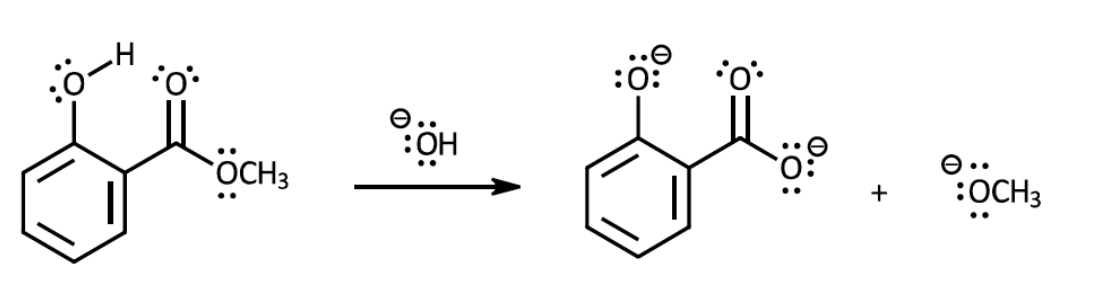
Transcribed Image Text::0-4
OCH3
HO:
:OCH3
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning