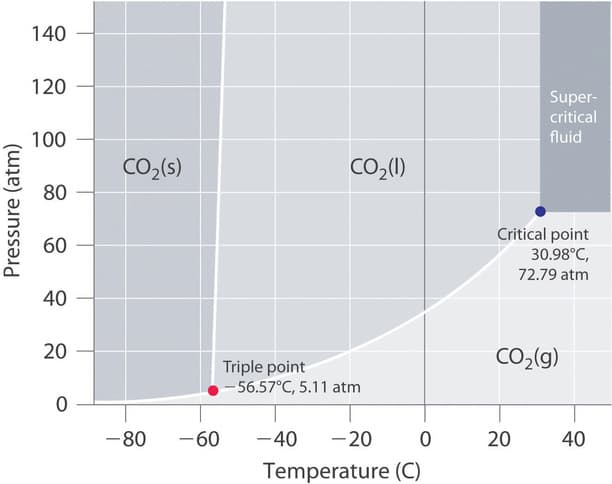
Using the phase diagram of carbon dioxide, describe the following conditions as stated below. 1. What happens when the follovving changes are made in a CO2 sample, which initially records at 1 atm. and -60°C? (a) Pressure increases at a constant temperature to 80 atm. (b) Temperature increases from -60°C to -18°C at constant 80 atm.pressure.
Using the phase diagram of carbon dioxide, describe the following conditions as stated below. 1. What happens when the follovving changes are made in a CO2 sample, which initially records at 1 atm. and -60°C? (a) Pressure increases at a constant temperature to 80 atm. (b) Temperature increases from -60°C to -18°C at constant 80 atm.pressure.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.3E
Related questions
Question
100%
Using the phase diagram of carbon dioxide, describe the following conditions as stated below.
1. What happens when the follovving changes are made in a CO2 sample, which initially records at 1 atm. and -60°C?
(a) Pressure increases at a constant temperature to 80 atm.
(b) Temperature increases from -60°C to -18°C at constant 80 atm.pressure.
Transcribed Image Text:140
120
Super-
critical
100
fluid
CO,(s)
CO,(1)
80
Critical point
30.98°C,
60
72.79 atm
40
20
CO2(g)
Triple point
- 56.57°C, 5.11 atm
-80
-60
-40
- 20
20
40
Temperature (C)
Pressure (atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning