Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 24CR: Define a saturated solution. Does saturated mean the same thing as saying the solution is...
Related questions
Question
Using the solubility curve in the second picture answer the following questions.
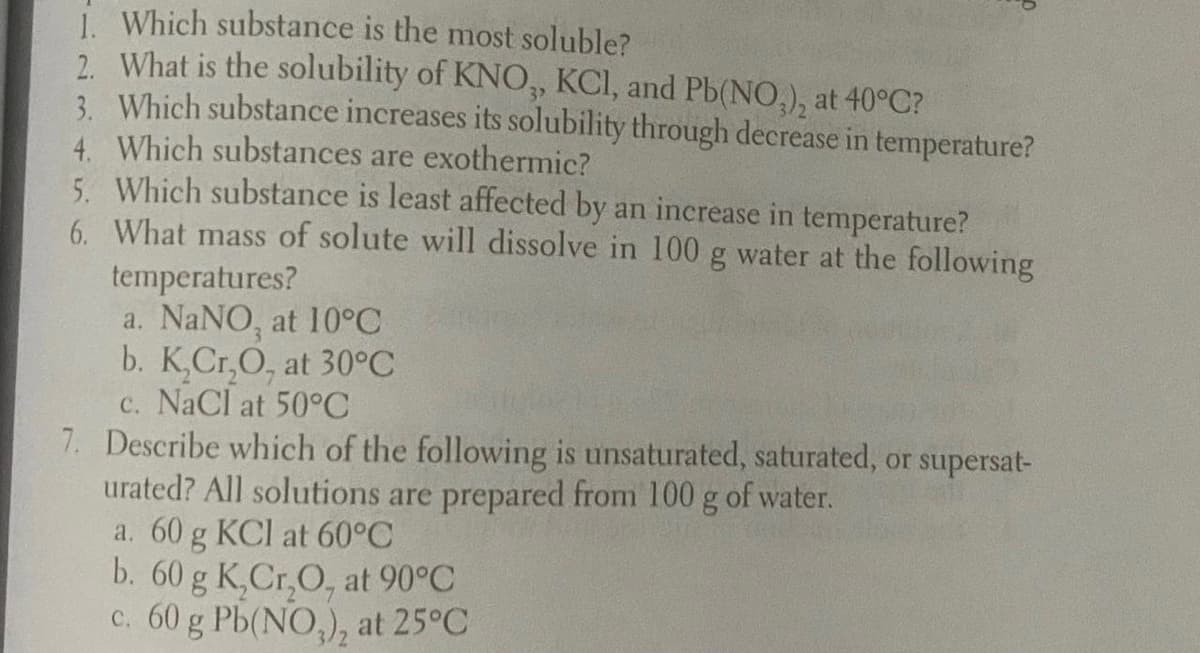
Transcribed Image Text:i. Which substance is the most soluble?
2. What is the solubility of KNO, KCI, and Pb(NO,), at 40°C?
3. Which substance increases its solubility through decrease in temperature?
4. Which substances are exothermic?
5. Which substance is least affected by an increase in temperature?
6. What mass of solute will dissolve in 100 g water at the following
temperatures?
a. NaNO, at 10°C
b. K,Cr,O, at 30°C
c. NaCl at 50°C
7. Describe which of the following is unsaturated, saturated, or supersat-
urated? All solutions are prepared from 100 g of water.
a. 60 g KCl at 60°C
b. 60 g K,Cr,O, at 90°C
c. 60 g Pb(NO,), at 25°C
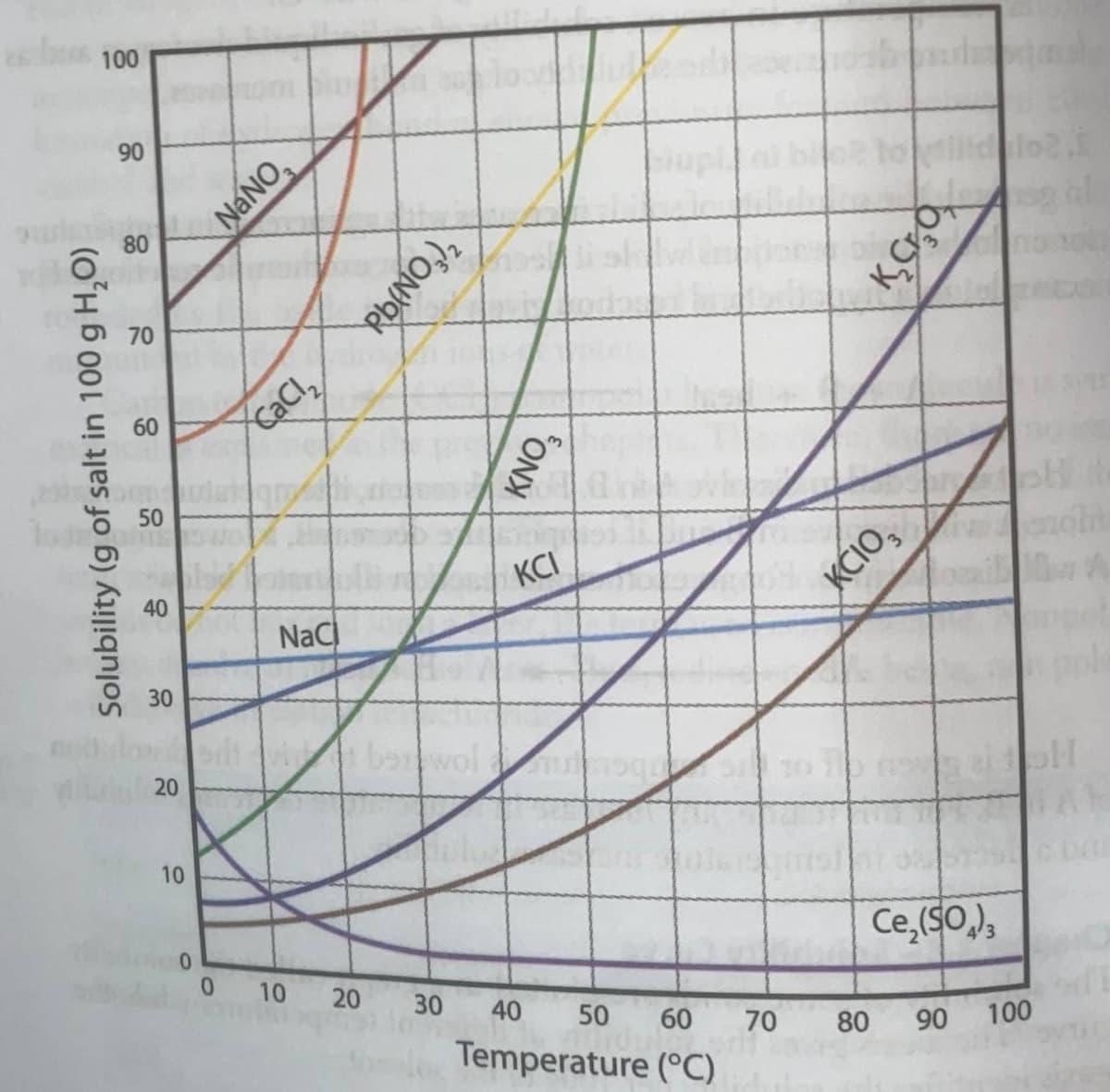
Transcribed Image Text:100
90
NaNO,
80
70
60
cacl
50
40
KCI
NaC
KĆIO,
30
10
Ce,(SO,),
10
30
40
50
60
70
Temperature (°C)
90
100
80
ON
20
20
Solubility (g of salt in 100 g H,O)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning