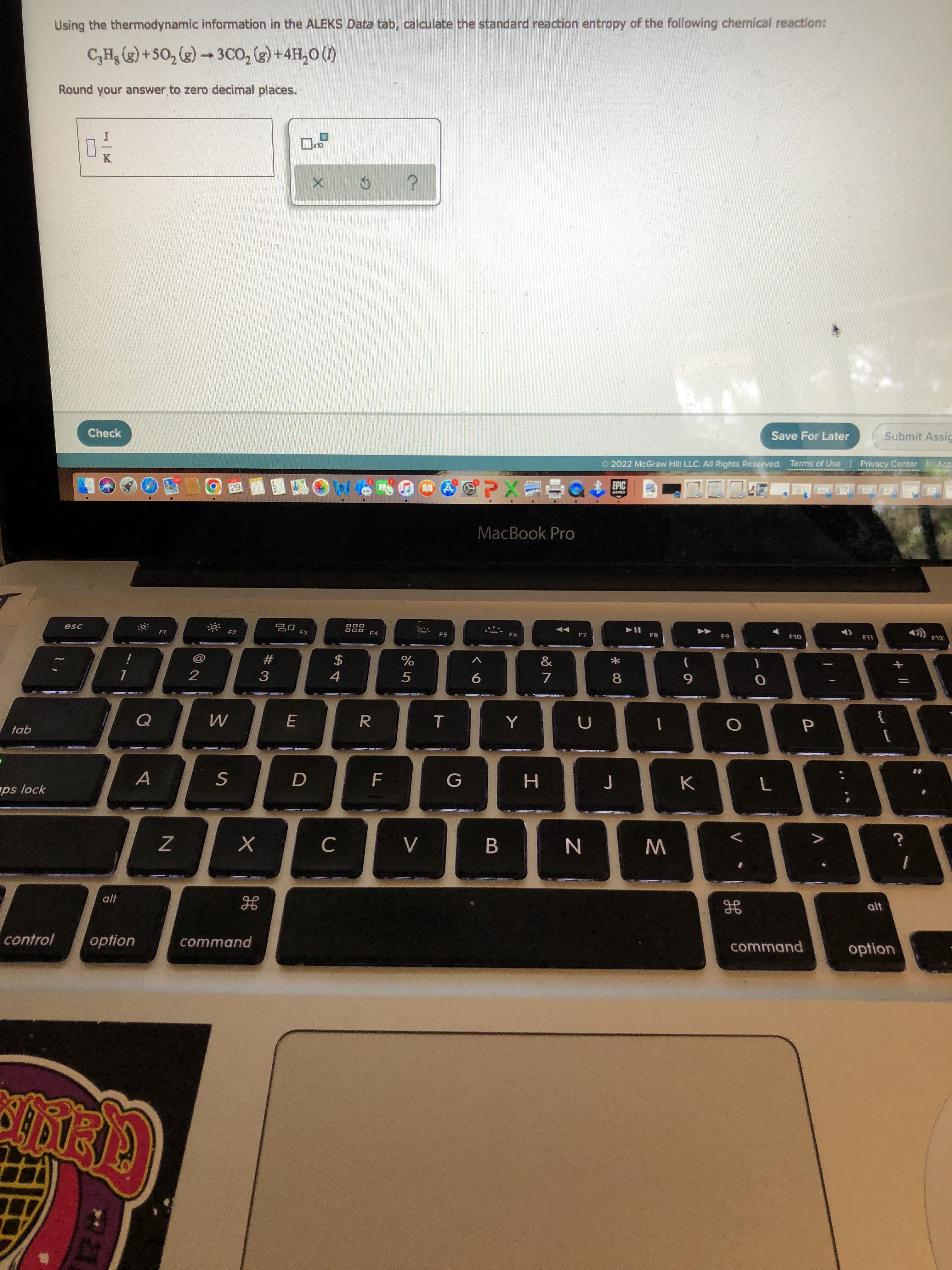
Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: C₂H₂(g) +50₂ (g) →3CO₂ (g) + 4H₂O(A). Round your answer to zero decimal places. 0-- ? x10 X Ś
Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: C₂H₂(g) +50₂ (g) →3CO₂ (g) + 4H₂O(A). Round your answer to zero decimal places. 0-- ? x10 X Ś
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section18.7: The Interplay Of Kinetics And Thermodynamics
Problem 2.2ACP: It has been demonstrated that buckminsterfullerene (C60), another allotrope of carbon (Section 2.3),...
Related questions
Question
Transcribed Image Text:tab
ps lock
control
Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction:
C₂Hg (g) +50₂ (g) → 3CO₂(g) + 4H₂O(1)
Round your answer to zero decimal places.
Ś ?
Save For Later
Submit Assig
Check
wwwwwwwww
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acc-
EPIC
GAMES
F5
F6
F7
F8
F9
F12
F10
&
(
1888888
6
7
9
T
Y
U
I
O
P
B
J
alt
option
esc
D
Q
A
alt
option
21
F1
Z
@2
W
S
79
X
H
command
ㅁㅁ
#3
E
F3
D
X
WW
$
4
C
***
DOD
DOO
F4
R
F
%
5
(1)
V
G
MacBook Pro
:
B
H
N
M
K
H
L
command
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning