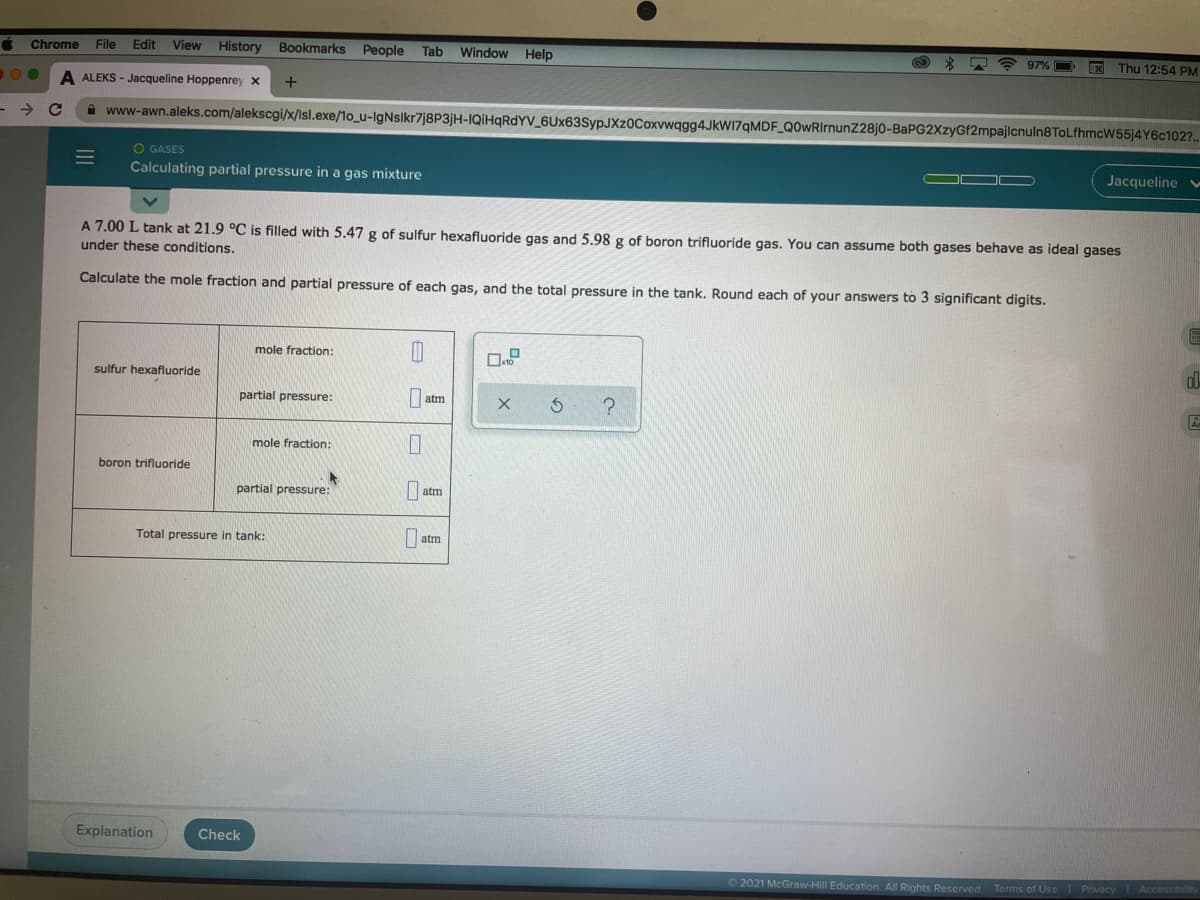
V55)4 Y6C102?. O GASES Calculating partial pressure in a gas mixture Jacqueline v A 7.00 L tank at 21.9 °C is filled with 5.47 g of sulfur hexafluoride gas and 5.98 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: sulfur hexafluoride partial pressure: atm mole fraction: boron trifluoride partial pressure: atm Total pressure in tank: | atm
V55)4 Y6C102?. O GASES Calculating partial pressure in a gas mixture Jacqueline v A 7.00 L tank at 21.9 °C is filled with 5.47 g of sulfur hexafluoride gas and 5.98 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: sulfur hexafluoride partial pressure: atm mole fraction: boron trifluoride partial pressure: atm Total pressure in tank: | atm
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 60P
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View History
Bookmarks People
Tab
Window Help
97% O
Thu 12:54 PM
A ALEKS - Jacqueline Hoppenrey x
A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7qMDF_Q0wRIrnunZ28j0-BaPG2XzyGf2mpajlcnuln8ToLfhmcW55j4Y6c102?..
O GASES
Calculating partial pressure in a gas mixture
Jacqueline v
A 7.00 L tank at 21.9 °C is filled with 5.47 g of sulfur hexafluoride gas and 5.98 g of boron trifluoride gas. You can assume both gases behave as ideal gases
under these conditions.
Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits.
mole fraction:
sulfur hexafluoride
partial pressure:
atm
mole fraction:
boron trifluoride
partial pressure:
atm
Total pressure in tank:
atm
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy
Access
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning