Vapor pressure (mm Hg) 900 800 700 600 500 400 300 200 100 0. Carbon disulfide 10 20 30 °C Methano 40 Ethandl Heptane 50 Temperature (°C) 60 70 80 90 100 110 From the plot of vapor pressures vs temperature above, estimate the normal boiling point of carbon disulfide.
Vapor pressure (mm Hg) 900 800 700 600 500 400 300 200 100 0. Carbon disulfide 10 20 30 °C Methano 40 Ethandl Heptane 50 Temperature (°C) 60 70 80 90 100 110 From the plot of vapor pressures vs temperature above, estimate the normal boiling point of carbon disulfide.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 21PS: Equilibrium vapor pressures of benzene, C6H6, at various temperatures are given in the table. (a)...
Related questions
Question
Aa.72.
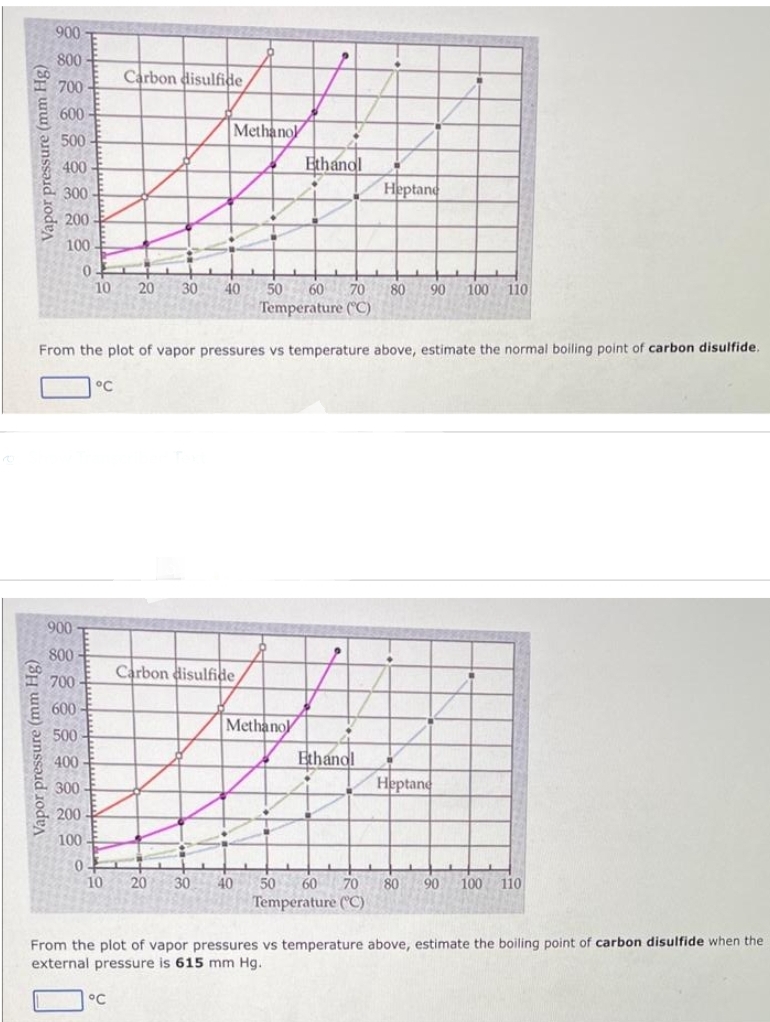
Transcribed Image Text:Vapor pressure (mm Hg)
900
Vapor pressure (mm Hg)
800
700
600
500
400
300
200
100
0
900-
800
700
600
500
400
300
200
100
0
10 20 30
Carbon disulfide
°C
10 20 30
°C
Methanol
40
From the plot of vapor pressures vs temperature above, estimate the normal boiling point of carbon disulfide.
Carbon disulfide
40
P
Methanol
Ethandl
50
Temperature (°C)
60 70 80
Heptane
P
Ethanol
90 100 110
Heptane
50 60 70 80 90 100 110
Temperature (°C)
From the plot of vapor pressures vs temperature above, estimate the boiling point of carbon disulfide when the
external pressure is 615 mm Hg.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax