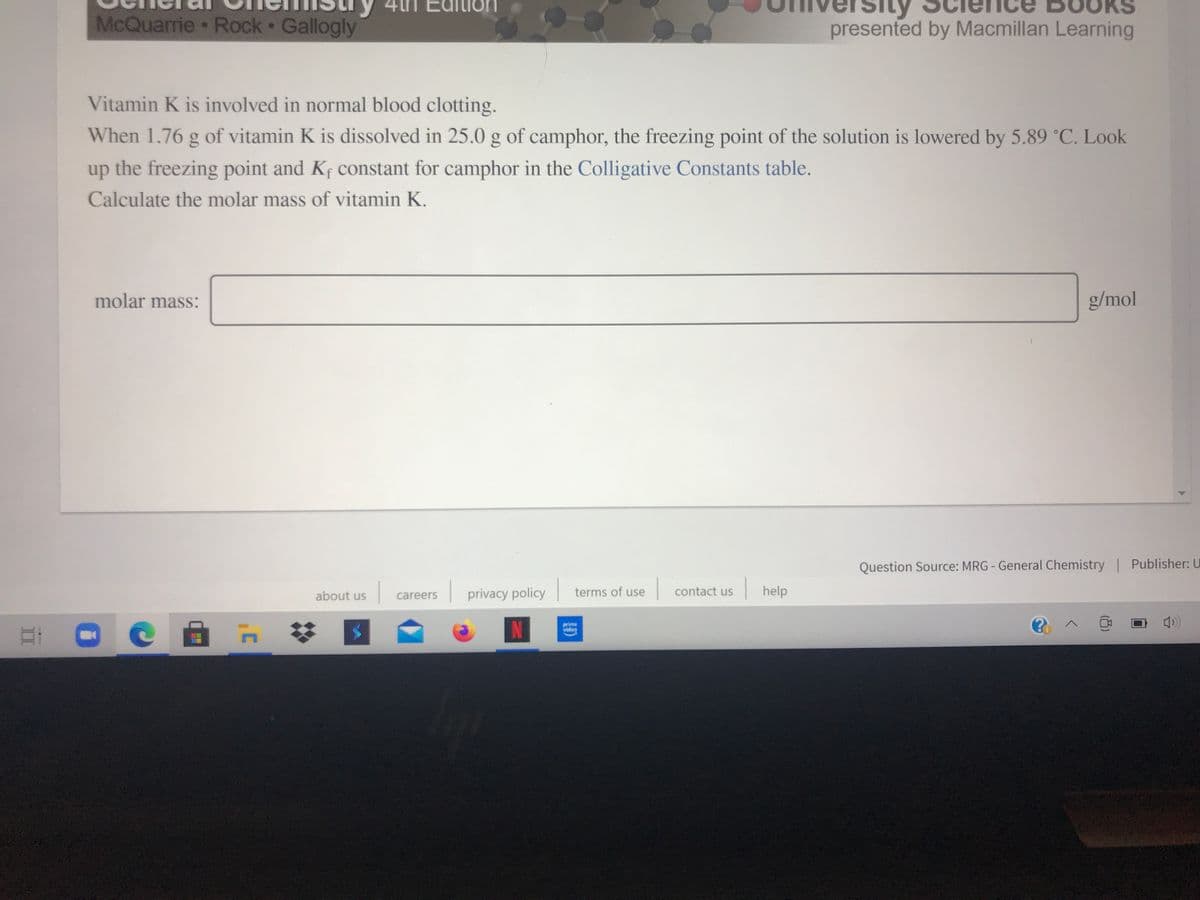
Vitamin K is involved in normal blood clotting. When 1.76 g of vitamin K is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 5.89 °C. Look up the freezing point and Kf constant for camphor in the Colligative Constants table. Calculate the molar mass of vitamin K. molar mass: g/mol
Vitamin K is involved in normal blood clotting. When 1.76 g of vitamin K is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 5.89 °C. Look up the freezing point and Kf constant for camphor in the Colligative Constants table. Calculate the molar mass of vitamin K. molar mass: g/mol
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 68QAP: Consider two solutions at a certain temperature. Solution X has a nonelectrolyte as a solute and an...
Related questions
Question
Transcribed Image Text:4th
McQuarrie Rock Gallogly
presented by Macmillan Learning
Vitamin K is involved in normal blood clotting.
When 1.76 g of vitamin K is dissolved in 25.0 g of camphor, the freezing point of the solution is lowered by 5.89 °C. Look
up the freezing point and Kf constant for camphor in the Colligative Constants table.
Calculate the molar mass of vitamin K.
molar mass:
g/mol
Question Source: MRG - General Chemistry | Publisher: U
| help
about us
careers
privacy policy
terms of use
contact us
N
prime
video
II
Expert Solution
Step 1
Given, the mass of solute vitamin K = 1.76g
Mass of solvent camphor = 25.0g
Depression in freezing point = 5.89oC.
Kf of camphor = 37.7oC/m
Freezing point of camphor =178.4oC.
What is the molar mass of vitamin K?
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning