Volume of sulfuric acid in sample (ml) Initial burette reading (ml) Final burette reading (ml) Total volume of 0.25 mol/L KOH added (ml) Average Volume of KOH added (mL) Trial 1 25.0 0.0 19.8 Trial 2 25.0 19.8 39.4 Trial 3 25.0 0.3 20.0
Q: The following observations are obtained after a D-hexose was made to react with several reagents:…
A:
Q: Suppose the following exchange of water reactions were carried out by an associative mechanism…
A:
Q: What is the product to the reaction below? NO₂ He at NO₂ b. Select one: Oa. Structure a O b.…
A:
Q: 7. O A. Rearrangement reaction. OB. Elimination reaction. O C. Isomerition reaction. OD. Addition…
A: Correct option is C isomerisation reaction Explanation: Isomerisation reaction involve…
Q: Question 15 of 31 Provide the correct IUPAC name for the skeletal (line-bond) structure shown here.…
A:
Q: Describe how you would prepare the following. Name reactants and products and rx conditions. a)…
A:
Q: Almost all thermoset are .38 ................moulded by A. injection moulding. O B. Comerssion. O C.…
A: 38) Almost all thermoset are moulded by compression. compression moulding is used for all…
Q: When the number of protons changes, the identity of the element changes. Many actinides are formed…
A: A question based on nuclear chemistry that is to be accomplished.
Q: 4000 100 38898 IR Spectrum (liquid film) 80 MMMMM % of base peak 10 3000 43 40 13C NMR Spectrum (50…
A:
Q: Suppose you are measuring the mass of a solid sample on a balance using a weigh boat. You record the…
A: given, The mass of weigh boat = 3.451 g. Mass of weigh boat and sample =…
Q: 29- The hybridization of carbon atom in alkene is. OA-30+1 πt. n. OB-40 OC-20+21 4 30 - The…
A: 29. Hybridization of carbon in Alkene is 3 Sigma + 1 pie bond , SP2 hybridized.
Q: Write the balanced chemical equation for the reaction shown. Carbon is represented by a black…
A:
Q: At a certain temperature, the reaction below has an equilibrium constant of 42. Which of the…
A:
Q: General formula of aromatic - 224 .compounds are A - CnH2n. O B - CnHn+2. O C- CnH2n-2. O
A: General formula for the aromatic compounds is given below.
Q: Arrange and give reason the following sets of compounds in order of: Increasing acidity Ph CH₂ H₂C…
A:
Q: 38. Fluorinating water is traditionally done to protect the enamel on teeth. One method used to…
A:
Q: Name three observations that might indicate that a chemical reaction has occurred. Write a…
A: Since you have asked a question with multiple sub-parts, as per our company guidelines we are…
Q: 10 Calcium oxide (CaO), also called quicklime, is an extremely valuable inorganic substance used in…
A: Calcium carbonate, CaCO3(s) undergoes decomposition reaction to form CaO(s) and CO2(g)
Q: 16. Strong bonding in organic chemistry is. O A. Covalent bonding. OB. Hydrogen bonding. O C. Van…
A:
Q: (35), - (25), (P), - (OP), = =
A: ->The above given equation can be proved with the help of thermodynamics equations.
Q: Based on these results, whats the equivalence point? and NaOH molarity for both trials if my…
A: To determine the equivalence point of the given titration between the NaOH and the citric acid.
Q: 3-chloro-6,7-dimethyloct-1-ene and (E)-1-chloro-5,6-dimethylhept-2-ene are primary and secondary…
A:
Q: method
A: We know that potassium chromate is a metal chromate indicator.
Q: 4) An oxidizing agent is a reactant that... A. accepts electrons and oxidizes another reactant B.…
A: Given, In the redox reaction, participate reactants one can act as oxidizing agent another act as…
Q: What is the advantage of using automated peptide synthesis? A) No side products are formed. B) Ease…
A: Automated peptide synthesis is a fast and easy to synthesize many peptides simultaneuosly. It…
Q: Q7. What is the correct Lewis structure for the molecule in the box, including the formal charge(s),…
A:
Q: IR Spectrum (liquid film) 4000 100 80 60 % of base peak 29 3000 57 74 40 80 13C NMR Spectrum (50.0…
A: Number of signals in 1H NMR = number of sets of protons in different electronic environment
Q: .Network polymer structure is .15 A. Branching. O B. Liner. O C. Cross-link. O Strong bonding in…
A:
Q: A 267−mL benzene solution containing 2.27 g of an organic polymer has an osmotic pressure of 7.37 mm…
A: 267 mL of benzene solution contains 2.27 g of an organic polymer has an osmotic pressureof 7.37 mm…
Q: 4000 100 80 60 8 8 40 IR Spectrum (liquid film) 20 % of base peak 3000 59 2000 115 87 11 80 40 13C…
A:
Q: Where did you get 5.8166
A: See the quadratic equation, 4.8166 = x/(2.4-x) 4.8166 × (2.4-x) = x 4.8166 × 2.4 - 4.8166x = x…
Q: Consider an E2 reaction between (2-bromoethyl)cyclohexane and base potassium tert-butoxide. Describe…
A:
Q: 1. 2. 3. . ما D ۸۰۸ zor 4. CI 5. CI CI ه CI
A:
Q: 35. The polymer is if heated too much, they burn is. A. Thermoset. B. Thermoplastic.
A: Thermo plastics soften on heating whereas thermosetting plastics do not soften on heating.…
Q: Complete the following bromination mechanism that has already been initiated with light Hot Use…
A:
Q: .The hybridization of carbon atom in alkane is - 24 A-Sp³ 30+ 1. B - Sp³ 40 C − Sp3 2 ơ + 2 . A O BO…
A:
Q: Look up the stereoselectivity model for this reaction and demonstrate how it is used to predict…
A: Ingredients in AD-mix-β and their functions Potassium osmate K2OsO2(OH)4 as the source of Osmium…
Q: To convert 20 g of ice at -5oC to 120oC to steam you need _______ cal of energy?
A:
Q: 8. ******* Which involve large number of organic reaction donot involve and free radicals OA.…
A: Organic reactions are proceeded by many mechanisms like addition, substitution, elimination, etc.…
Q: A) Identical molecules B) Stereoisomers C) Constitutional isomers D) Isotopes E) Neither identical…
A: These both molecules are stereo isomers. 1st one is trans isomer and 2nd one is cis isomer. Cis and…
Q: Standard Reduction Potentials Half-Reaction E° (V) Mg2+ + 2e → Mg -2.37 Pb2+ + 2e → Pb -0.13…
A:
Q: Consider the structure of the peptide below and answer the questions that follow. CH₂ OH Re choose…
A: Here we are required to find N terminal amino acid
Q: 17. ********** melted. OA. Thermoplastics *********** are cured into permanent shape is Cannot be…
A:
Q: The following observations are obtained after a D-hexose was made to react with (1) The reactions of…
A:
Q: CHO H-OH H-OH CH₂OH 1. NaCN/HCI 2. H₂/Pd/BaSO4 3. H₂O/H
A:
Q: HClO4(aq)+NaOH(aq)→ Express your answer as a chemical equation. Identify all of the phases in your…
A: The acid react with base to form salt and water , called as neutralization reaction.
Q: How many kg are in 34g?
A: Given, The mass of a substance = 34 grams Number of Kg in 34 g is:
Q: Examine the geometry of the species in the window below. Use the atom geometry to determine the…
A: Here structure of an organic compound is given, which contains carbon,hydrogen and oxygen atom.…
Q: Given that there are 1000mg in one gram, how many mg are in 0.0045g?
A: Here we are required to convert 0.0045g to milligrams.
Q: N ΟΝ OU G LC G b app.101edu.co Tube Maps n H3C-CH₂ H3C 0 A In T H 2 bA OC Question 7 of 31 The…
A:
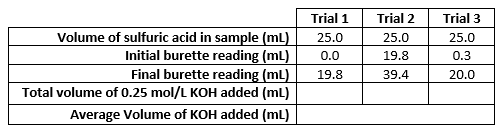
A student wants to determine the unknown concentration of a sample of sulfuric acid.
She performs a titration between her sample of sulfuric acid and potassium hydroxide and collects the data below.
Determine the unknown concentration of sulfuric acid.
Step by step
Solved in 4 steps

- Sucrose solution for experiment 1: (Question a-c)Prepare a solution of sucrose by accurately weighing 9.0 – 9.25 g of sucrose into a 100mLbeaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until allcrystals dissolve.NaCl solution for experiment 2: (Question d-f)Prepare a solution of NaCl by accurately weighing approximately 1.5 g of NaCl into a 100 mLbeaker / conical flask. Record the mass of the sample. Add 25 mL distilled water. Mix until allcrystals dissolve.MgCl2٠6H2O solution for experiment 2: (Question d-f)Prepare a solution of magnesium chloride by accurately weighing approximately 3.6 g ofMgCl2٠6H20 into a 100 mL beaker / conical flask. Record the mass of the sample. Add 25 mLdistilled water. Mix until all the crystals dissolveYou collected the following data from a titration experiment using a 0.118M standardized NaOH solution to titrate a 26.65 mL solution with an unknown Molarity concentration (M) of sulfuric acid (H2SO4). Initial Burette Reading (mL) Final Burette Reading (mL) Vol Delivered (mL) Acid Concentration (M) Trail 1 0.15 19.47 ? ? Trail 2 0.18 17.3 Trail 3 0.17 18.05 State the volume delivered and acid concentration for just the first trail. Use significant digits.Please put your observations in data table part 2 and answer the post lab questions(1-5) AND 6 A AND B and for number 7 ONLY DO THE EQUATIONS FOR 11 12 AND 13 I MARKED ON THE WORKSHEET BUT ONLY DO EQUATION FOR HCI WITH CUSO4 AGNO3 AND FE2SO4 I KNOW I DID IT BUT I MAY BE WRONG
- Why is NaOH(s) to be taken out and weighed only when you are ready to make measurements? Group of answer choices NaOH does not need special treatment. NaOH reacts violently so is prepared last. NaOH is hygroscopic NaOH burns holes in the weighing paper.A student creates a calibration plot for serial dilutions of Cu(NO3)2. A graph of concentration in M (x-axis) versus absorbance (y-axis) gives a linear trendline of y = 0.345x - 0.11. Copper(II) nitrate has a solubility of 83.5 g in 100 g of water. Calculate the percent error in the student's concentration.Convert each "b" value from the three Graphs ln(Conc) to the Initial Concentration. Graph Two: y= -0.0048x -11.125 Graph Four: y= 161824x - 13.326 Graph Five: y= -0.0189x -10.903 **Note these are the y= mx-b equation from the linear graph, In(concentration) vs time. I don't know how to convert natural log value such as -11.125 to the initial concentration.
- Assignment: This is an individual activity. After studying the lesson and watching all the videos, answer the following questions. Answer in not more than 3 sentences per question. 1. Discuss the a) direct method of weighing b) indirect weighing using the analytical balance. 2. Explain how suction filtration is carried out in the laboratory. Give the important points that must be observed in doing the process. 3. Explain why it is important to wash the precipitate. Can distilled water be used to wash all types of precipitates? 4. Discuss how to use a desiccator. When should a desiccant be replaced?An environmental scientist obtained the following replicate concentration measurements of methane (CH4) gas at a landfill site: Replicate Measurements CH4 Concentration (mg/m3) 1.0 0.1571 2.2 0.2031 3.0 0.1914 4.0 0.2103 Calculate % error when the scientific community accepts 0.2102 as true value.Data obtained: Chips # of extractions Chips' weight (g) Fat weight (g) Regular 3 20.043 6.745 3 20.187 6.438 3 20.198 7.451 Low fat 3 19.456 3.982 3 20.072 4.547 3 20.192 4.589 Mass percent is a method of expressing the concentration of a substance in a mixture or element in a compound. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. The formula is: mass % of component= (mass of component / total mass) x 100% 1) Reproduce the following table, and use the data to determine the mass % of fat in the chips for each trial. 2) Show one example calculation. Chips Trial % by mass of fat Regular 1 2 3 Lowfat 1 2 3
- Which analytical separation method is best for recovering river bodies that are polluted by heavy metals and particulate matter. Explain your analytical process of choice.Part 1 – Preparation of CaCO3(s) The following is a procedure that was theoretically performed by a student. Read through the procedure and answer the questions below. A 10.0 mL graduated cylinder to measure 10.0 mL of a 1.00 M CaCl2 solution into an initially empty 50.0 mL beaker. A 50.0 mL graduated cylinder was then used to measure out 25.0 mL of 0.500 M K2CO3. This K2CO3 solution was then added to the beaker containing the CaCl2 The solution became cloudy, and the student concluded that a precipitate must have formed. Write a balanced chemical reaction below, including phases, and identify the chemical formula of the precipitate: Answer the questions below as part of Data Analysis. 1. What is the actual yield of the product? 2. Using stoichiometry, calculate the limiting reactant, and the theoretical yield (the mass of CaCO3 product) for the reaction. 3. Determine the percent yield of the reaction.You have finished two trials of the sample analysis for the experiment and you noticed that the burette is almost empty (~5 mL remaining). Should you add more titrant before the next sample analysis or not? Why or why not? Select one: YES. It will not affect the calculations and not cause error NO. It will affect the calculations and cause error Neither A nor B Cannot be determined

