1. Determination of the Ideal Gas Constant "R" using Carbón Dioxidê 10 °C. Volume of plastic bag 3700ml 768.0mm Hg Temperature Barometric pressure 4 (wet) 5 (wet) 33.6219 Trial 1 77,7219 69.62 Minitial Minal
1. Determination of the Ideal Gas Constant "R" using Carbón Dioxidê 10 °C. Volume of plastic bag 3700ml 768.0mm Hg Temperature Barometric pressure 4 (wet) 5 (wet) 33.6219 Trial 1 77,7219 69.62 Minitial Minal
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter13: Gases
Section: Chapter Questions
Problem 64A
Related questions
Question
Can u please help me to understand this problem please thank you
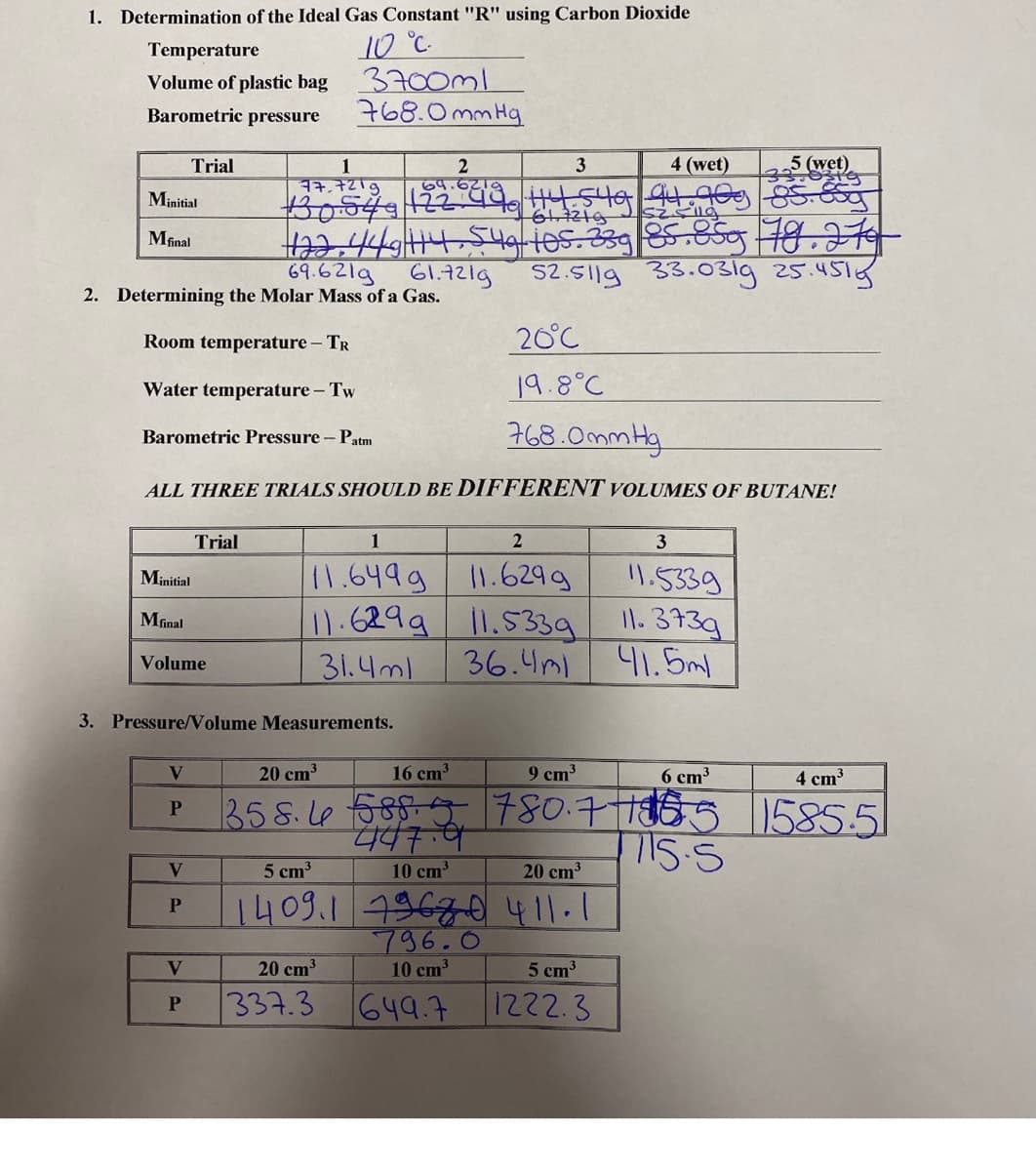
Transcribed Image Text:1. Determination of the Ideal Gas Constant "R" using Carbon Dioxide
10 °C.
3700ml
768.0mm Hg
Temperature
Volume of plastic bag
Barometric pressure
4 (wet)
5 (wet)
33
Trial
3
1
77,7219
2
69.621a
Minitial
ta2.44/14.549 les.239|85.859 78.714
69.621g 61.72lg
Mfinal
S2.511g
33.031g 25.451g
2. Determining the Molar Mass of a Gas.
Room temperature - TR
20°C
Water temperature - Tw
19.8°C
768.0mm Hg
Barometric Pressure - Patm
ALL THREE TRIALS SHOULD BE DIFFERENT VOLUMES OF BUTANE!
Trial
1
3
11.649g 1.6299
11.5339
11.6299 l1.539 l1.373g
36.4ml 41.5ml
Minitial
Mfinal
31.4ml
Volume
3. Pressure/Volume Measurements.
V.
20 cm
16 cm
9 cm3
6 cm3
4 cm3
358.le 585-5 780.775
1585.51
5 cm3
10 cm3
20 cm3
1409.1 19670411.1
796.0
10 cm3
V
20 cm3
5 cm
337.3
649.7
1222.3
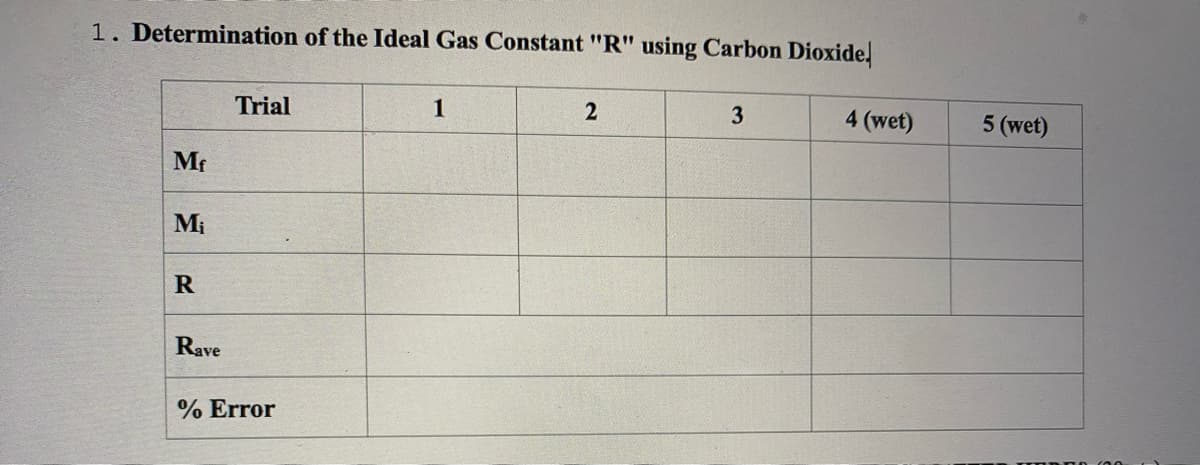
Transcribed Image Text:1. Determination of the Ideal Gas Constant "R" using Carbon Dioxidel
Trial
1
3
4 (wet)
5 (wet)
Mf
Mi
Rave
% Error
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning