Water undergoes a large change in density at 0 °C as it freezes to form ice. Calculate the percent change in density that occurs when liquid water freezes to ice at 0°C given that final value-initial value percent change x 100% %3D initial value Express as a percentage to two significant figures.
Water undergoes a large change in density at 0 °C as it freezes to form ice. Calculate the percent change in density that occurs when liquid water freezes to ice at 0°C given that final value-initial value percent change x 100% %3D initial value Express as a percentage to two significant figures.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 7E: 7.The word pour is commonly used in reference to liquids but not to solids or gases. Can you pour a...
Related questions
Question
Need help with part A
Transcribed Image Text:Course Home
1.202140X: Modified Mas x
envellum.ecollege.com/course.html?courseld3D16946408&OpenVellumHMAC=1795926163d:
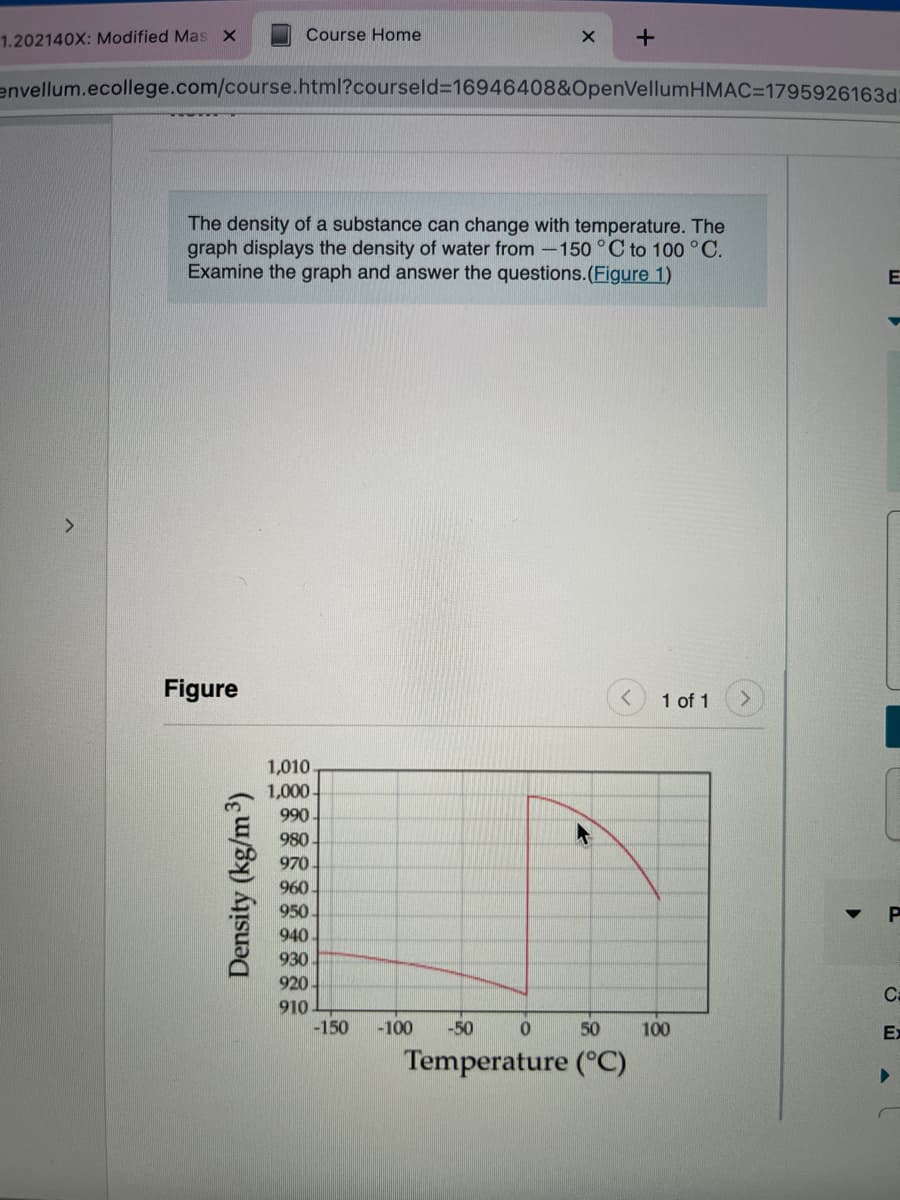
The density of a substance can change with temperature. The
graph displays the density of water from -150 ° C to 100 °C.
Examine the graph and answer the questions.(Figure 1)
Figure
1 of 1
1,010
1,000-
990
980
970
960
950
• P
940
930
920
Ca
910
-150
-100
-50
50
100
EX
Temperature (°C)
Density (kg/m3)
Transcribed Image Text:26163d38763aeeac4700b6d9d2e#10001
1 of 1
I Review I Constants I Periodic Table
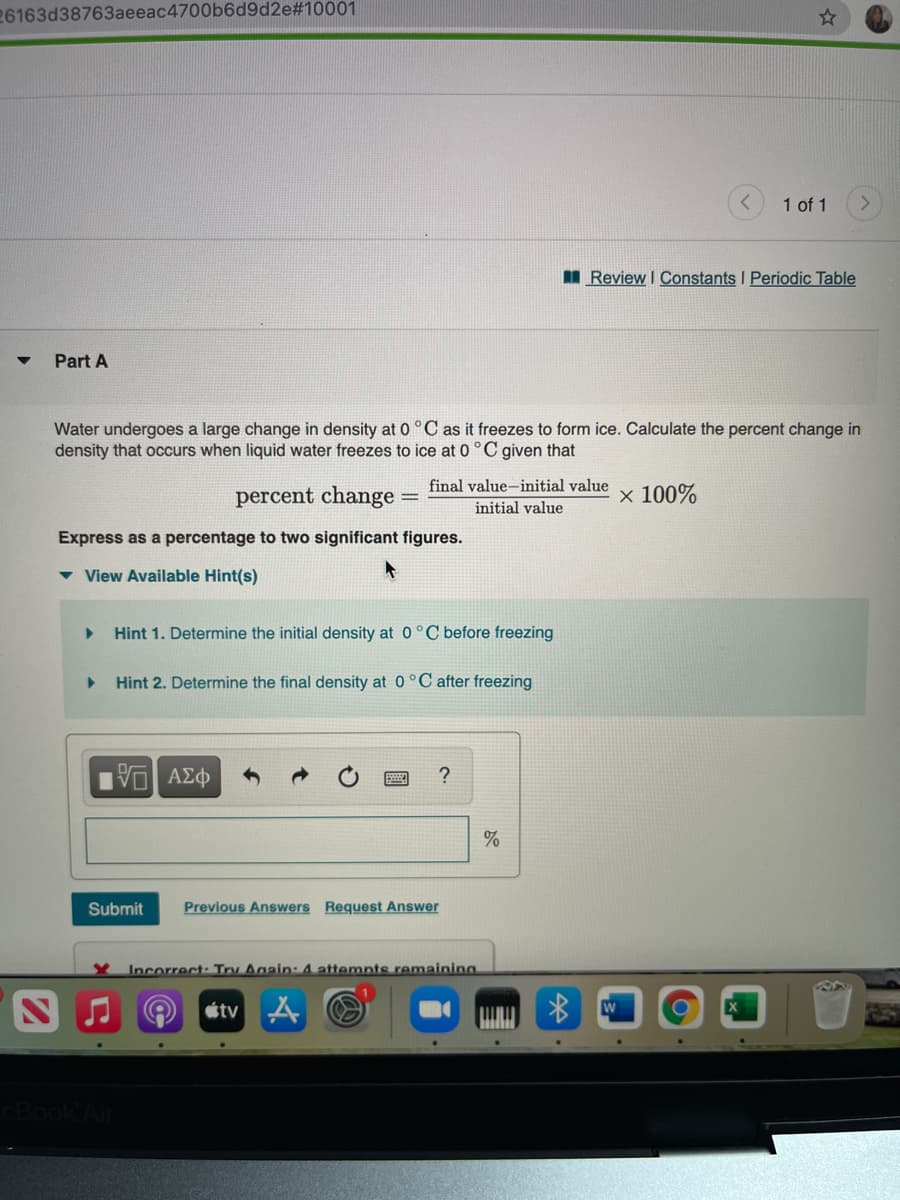
Part A
Water undergoes a large change in density at 0 °C as it freezes to form ice. Calculate the percent change in
density that occurs when liquid water freezes to ice at 0 °C given that
percent change:
final value-initial value
initial value
x 100%
Express as a percentage to two significant figures.
v View Available Hint(s)
Hint 1. Determine the initial density at 0°C before freezing
Hint 2. Determine the final density at 0°C after freezing
| να ΑΣφ
?
%
Submit
Previous Answers Request Answer
Incorrect: Try Again:4 attempts remainina
étv A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning