Consider the following equations. Which one relates the temperature on thermometer A to th temperature on thermometer B? в Bolling point of water 212 100 373 180 100 Freezing point of water 273 1) °A = °B + 273 2) °A -32 = 1.8 * OB 3) °A +32 = 1.8 * °B 4) °A = °R - 273
Consider the following equations. Which one relates the temperature on thermometer A to th temperature on thermometer B? в Bolling point of water 212 100 373 180 100 Freezing point of water 273 1) °A = °B + 273 2) °A -32 = 1.8 * OB 3) °A +32 = 1.8 * °B 4) °A = °R - 273
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter4: Introduction To Gases
Section: Chapter Questions
Problem 27E
Related questions
Question
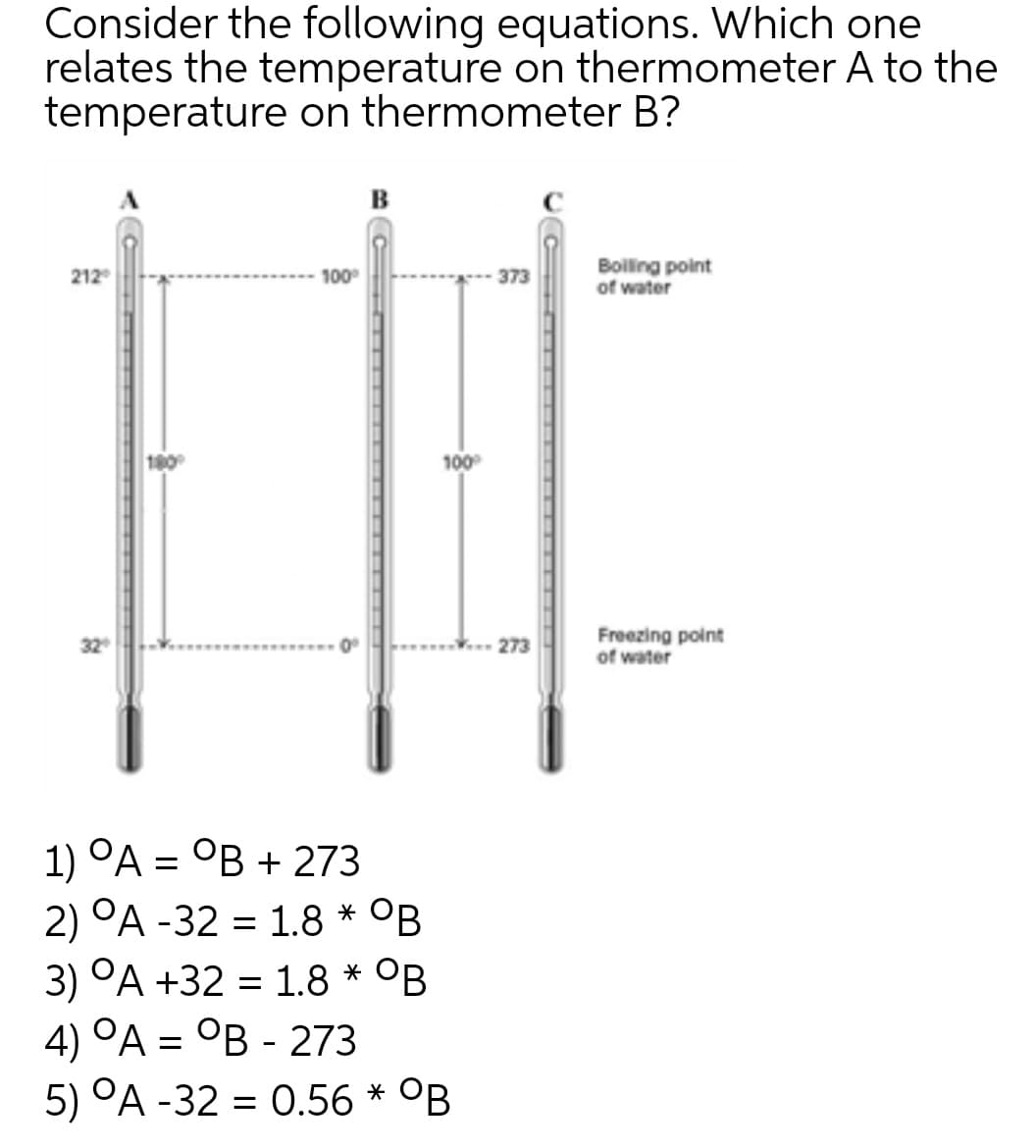
Transcribed Image Text:Consider the following equations. Which one
relates the temperature on thermometer A to the
temperature on thermometer B?
в
Boiling point
of water
212
100
373
100
Freezing point
of water
273
1) ОА — ОВ + 273
2) °A -32 = 1.8 * OB
3) °A +32 = 1.8 * °B
4) °A = °B - 273
5) °A -32 = 0.56 * °B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,