We know that for an ideal gas Cp,m = Cv,m + R. %3D The general relationship for any gas can be written, for 1 mole, as Cp,m = Cy,m + (Yn") a²VmT` k (equation-1) Where Vm is the molar volume (the volume of 1 mole of the gas, V/n). a is the coefficient of volume expansion and is given by: a = where the partial derivative of V with respect to T is calculated ƏT assuming P is constant And K is the coefficient of volume expansion and is given by: k = GO where the partial derivative of V with respect to P is calculated assuming T is constant. Note the minus in the definition. Prove that equation-1 reduces to the result derived in class for an ideal gas by calculating (A²V¼T\ k
We know that for an ideal gas Cp,m = Cv,m + R. %3D The general relationship for any gas can be written, for 1 mole, as Cp,m = Cy,m + (Yn") a²VmT` k (equation-1) Where Vm is the molar volume (the volume of 1 mole of the gas, V/n). a is the coefficient of volume expansion and is given by: a = where the partial derivative of V with respect to T is calculated ƏT assuming P is constant And K is the coefficient of volume expansion and is given by: k = GO where the partial derivative of V with respect to P is calculated assuming T is constant. Note the minus in the definition. Prove that equation-1 reduces to the result derived in class for an ideal gas by calculating (A²V¼T\ k
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 45QRT: At the critical point for carbon dioxide, the substance is very far from being an ideal gas. Prove...
Related questions
Question
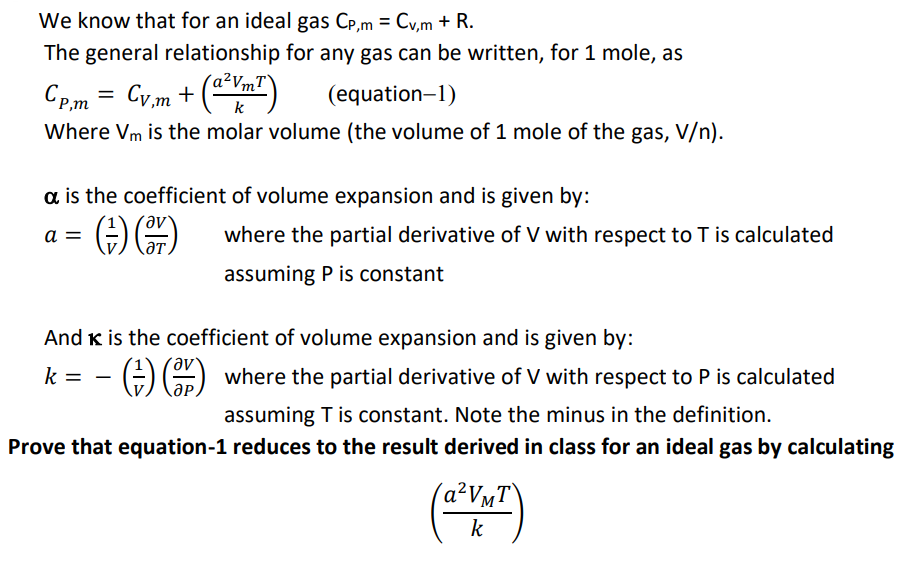
Transcribed Image Text:We know that for an ideal gas Cp,m = Cv,m + R.
%3D
The general relationship for any gas can be written, for 1 mole, as
Cp,m = Cy,m +
(Yn")
a²VmT`
k
(equation-1)
Where Vm is the molar volume (the volume of 1 mole of the gas, V/n).
a is the coefficient of volume expansion and is given by:
a =
where the partial derivative of V with respect to T is calculated
ƏT
assuming P is constant
And K is the coefficient of volume expansion and is given by:
k =
GO where the partial derivative of V with respect to P is calculated
assuming T is constant. Note the minus in the definition.
Prove that equation-1 reduces to the result derived in class for an ideal gas by calculating
(A²V¼T\
k
Expert Solution
Step 1
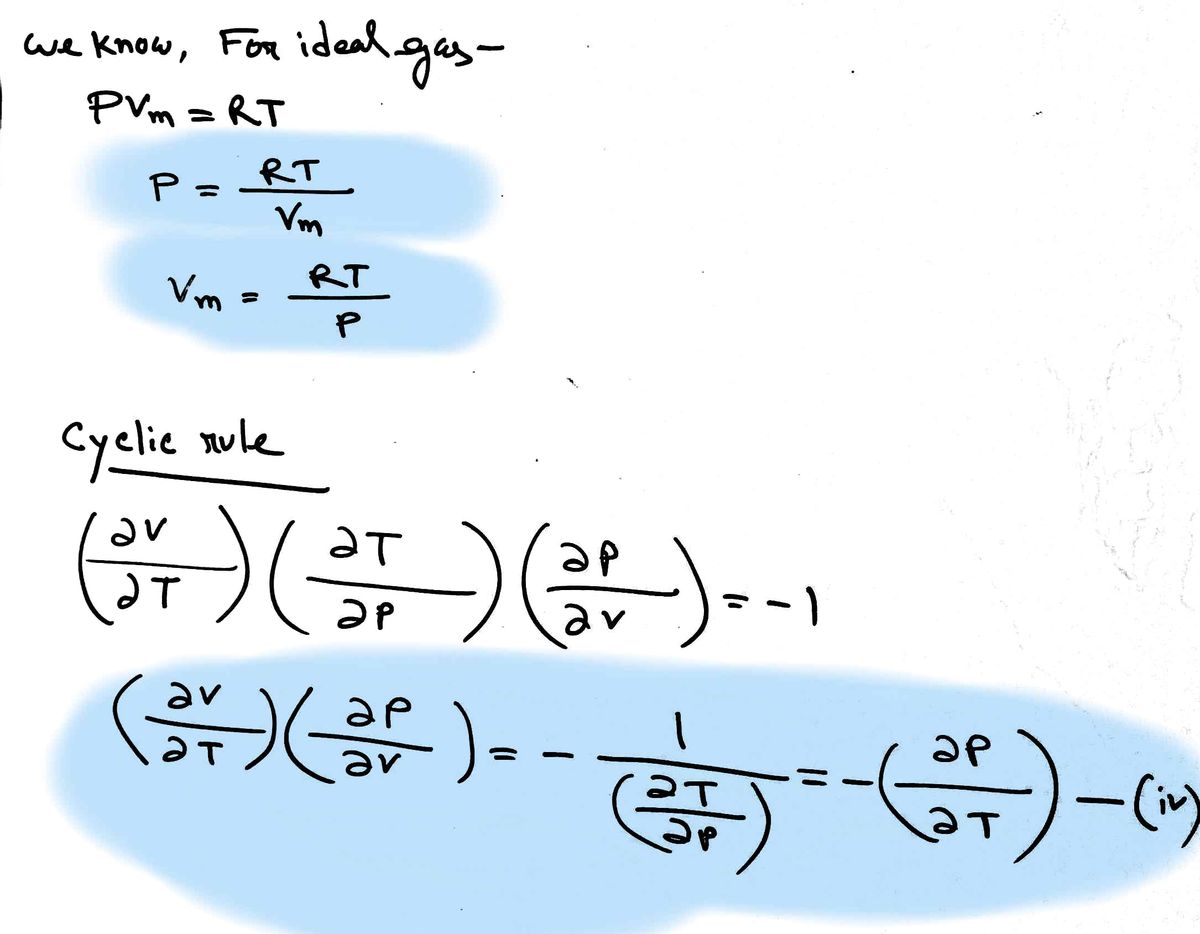
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,