What are the angles a and b in the actual molecule of which this is a Lewis structure? H H H OH Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes.
What are the angles a and b in the actual molecule of which this is a Lewis structure? H H H OH Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 41QAP: The uracil molecule is one of the bases in DNA. Estimate the approximate values of the indicated...
Related questions
Question
The first question was wrong please provide good answer
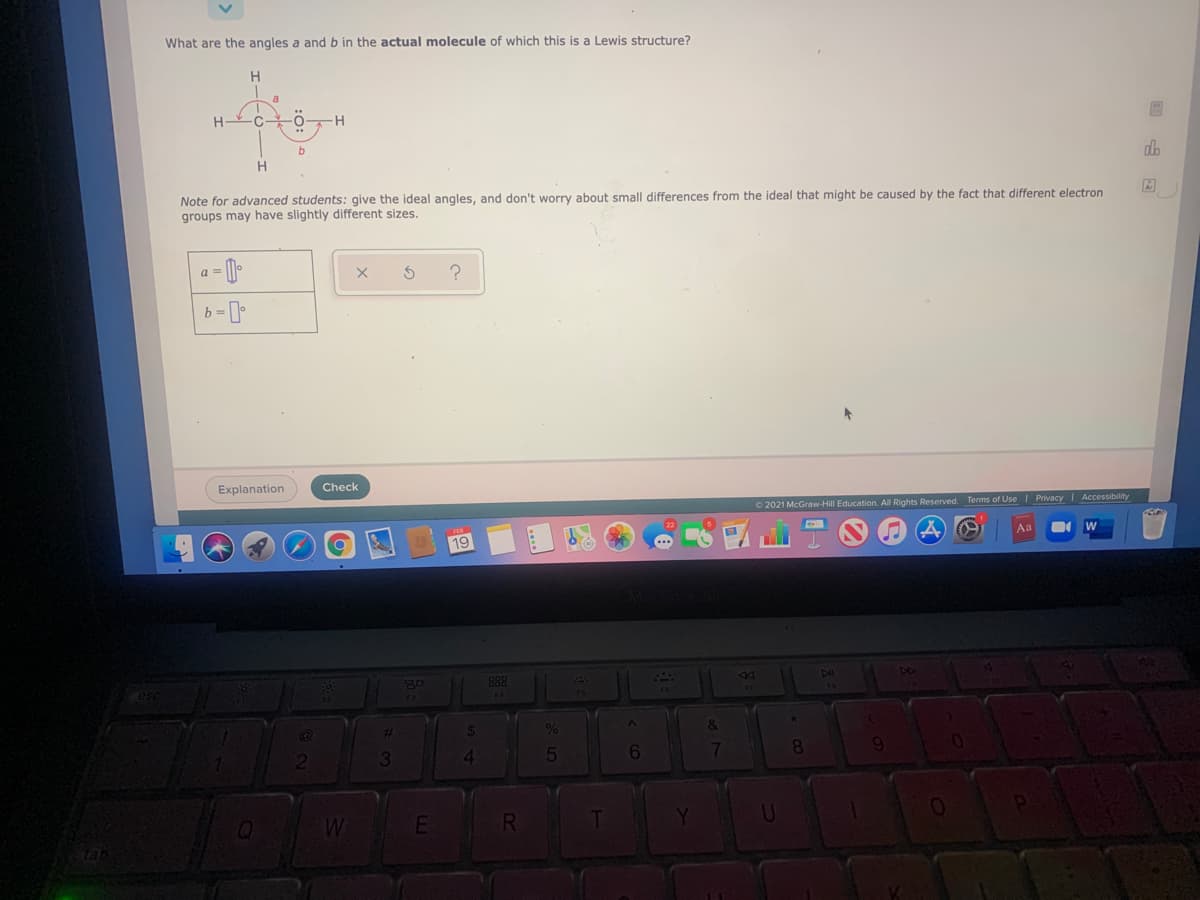
Transcribed Image Text:What are the angles a and b in the actual molecule of which this is a Lewis structure?
db
Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron
groups may have slightly different sizes.
a = [)
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of UseI Privacy I Accessibility
19
Aa
888
*.
%23
6.
8
Expert Solution
Step 1: Interpretation of the problem
In the given molecule:
Angle a: H-C-O
Angle b: C-O-H
So, angle a is around the central atom of carbon, and angle b.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
