
Concept explainers
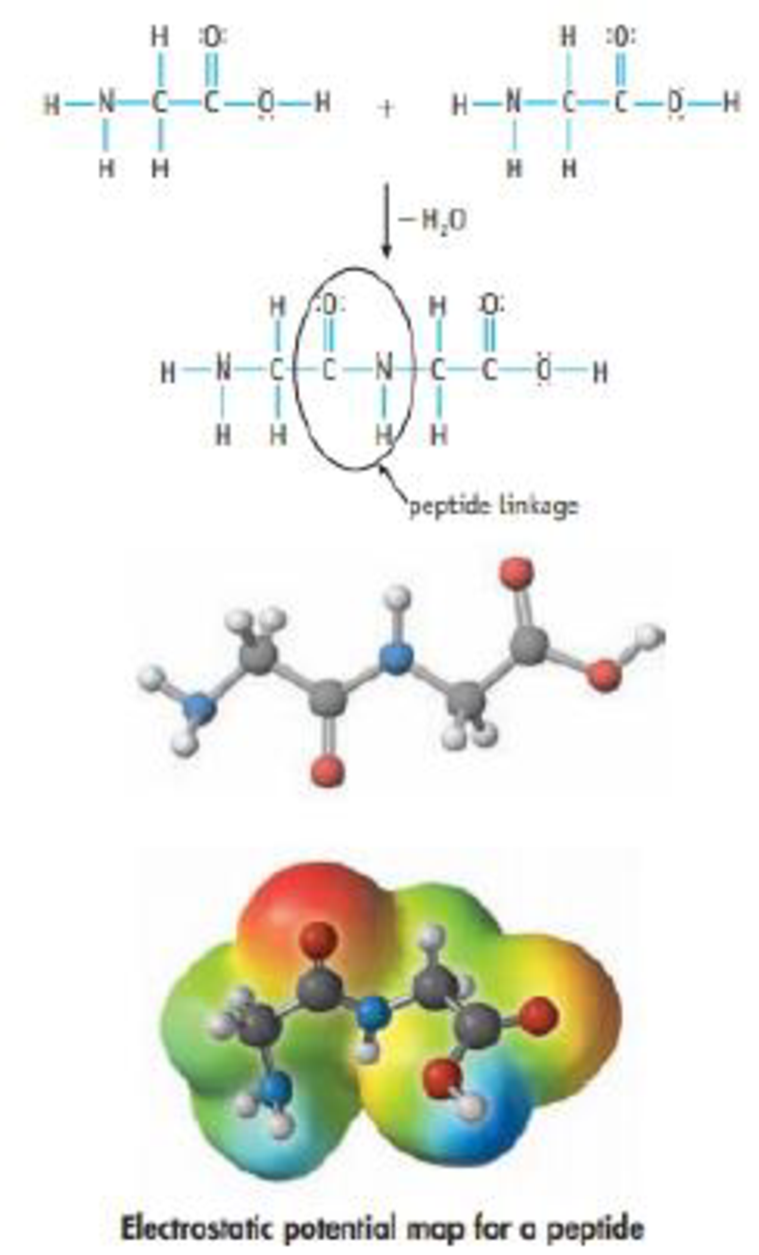
When two amino acids react with each other, they form a linkage called an amide group, or a peptide link. (If more linkage. are added, a protein or polypeptide is formed.)
(a) What are the hybridizations of the C and N atoms in the peptide linkage?
(b) Is the structure illustrated the only resonance structure possible for the peptide linkage? If another resonance structure is possible. compare it with the o ne shown. Decide which is the more important structure.
(c) The computer-generated structure shown here, which contains a peptide linkage, shows that this linkage is flat. This is an important feature of proteins. Speculate on reasons that the CO—NH linkage is planar. What are the sites of positive and negative charge in this dipeptide?
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Chemistry & Chemical Reactivity
- The uracil molecule is one of the bases in DNA. Estimate the approximate values of the indicated bond angles. Its skeleton (not its Lewis structure) is given below.arrow_forwardAcrylonitrile, C3H3N is the building mer Orlon. Its Lewis structure is What is the hybridization of nitrogen and of the three numbered carbon atoms?arrow_forward7.57 What observation about molecules compels us to consider the hybridization of atomic orbitals?arrow_forward
- In the hybrid orbital model, compare and contrast bonds with bonds. What orbitals form the bonds and what orbitals form the bonds? Assume the z-axis is the internuclear axis.arrow_forwardtrans-tetrazene (N4H4) consists of a chain of four nitrogen atoms with each of the two end atoms bonded to two hydrogen atoms. Use the concepts of steric number and hybridization to predict the overall geometry of the molecule. Give the expected structure of cis-tetrazene.arrow_forwardComplete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicated atoms.arrow_forward
- Give the hybridization for the O in OF2arrow_forwardWhat are the hybridizations of atoms 1 and 2 respectively in the following structure?arrow_forwardConsider the Lewis structure shown below. (a) Does the Lewis structure depict a neutral molecule or anion? If it is an ion, what is the charge on the ion? (b) What hybridizationis exhibited by each of the carbon atoms? (c) Arethere multiple equivalent resonance structures for the species?(d) How many electrons are in the π system of the species?arrow_forward
- Consider the molecule PF4Cl. (a) Draw a Lewis structure forthe molecule, and predict its electron-domain geometry.(b) Which would you expect to take up more space, a P¬Fbond or a P¬Cl bond? Explain. (c) Predict the molecular geometryof PF4Cl. How did your answer for part (b) influenceyour answer here in part (c)? (d) Would you expect the moleculeto distort from its ideal electron-domain geometry? Ifso, how would it distort?arrow_forwardGive the hybridization for the O in HaO+arrow_forwardBenzene is commonly regarded as a resonance hybrid ofthe two Kekule structures. but other possible structures canalso contribute. Draw three other structures in which thereare only covalent n bonds (al lowing for bonding betweensome non-adjacent C atoms) and two structures in whichthere is one ionic bond. Why may these structures be ignoredin simple descriptions of the molecule?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





