Draw the Lewis structures for the following molecules. For each molecule, determine its (a) electronic geometry, (b) number of nonbonding domains on the central atom, and (c) polarity of the molecule. Remember, that molecules that have zero nonbonding domains on the central atom AND have all of the outer atoms the same are NONPOLAR. Generally, molecules with different outer atoms or with more than one nonbonding domain are POLAR. However, these may be NONPOLAR ONLY IF the dipoles cancel each other due to a symmetrical arrangement. CIF3 (a) electronic geometry: -Select-- (b) number of nonbonding domains on central atom: Select-v (c) molecule polarity: -Select--V SO2 (a) electronic geometry: -Select- (b) number of nonbonding domains on central atom: -Select-- V (c) molecule polarity: -Select-v SF6 (a) electronic geometry: –Select– (b) number of nonbonding domains on central atom: -Select-- ♥ (c) molecule polarity: -Select-v IFS (a) electronic geometry: -Select- (b) number of nonbonding domains on central atom: -Select-v (c) molecule polarity: -Select-v
Draw the Lewis structures for the following molecules. For each molecule, determine its (a) electronic geometry, (b) number of nonbonding domains on the central atom, and (c) polarity of the molecule. Remember, that molecules that have zero nonbonding domains on the central atom AND have all of the outer atoms the same are NONPOLAR. Generally, molecules with different outer atoms or with more than one nonbonding domain are POLAR. However, these may be NONPOLAR ONLY IF the dipoles cancel each other due to a symmetrical arrangement. CIF3 (a) electronic geometry: -Select-- (b) number of nonbonding domains on central atom: Select-v (c) molecule polarity: -Select--V SO2 (a) electronic geometry: -Select- (b) number of nonbonding domains on central atom: -Select-- V (c) molecule polarity: -Select-v SF6 (a) electronic geometry: –Select– (b) number of nonbonding domains on central atom: -Select-- ♥ (c) molecule polarity: -Select-v IFS (a) electronic geometry: -Select- (b) number of nonbonding domains on central atom: -Select-v (c) molecule polarity: -Select-v
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 3CTQ
Related questions
Question
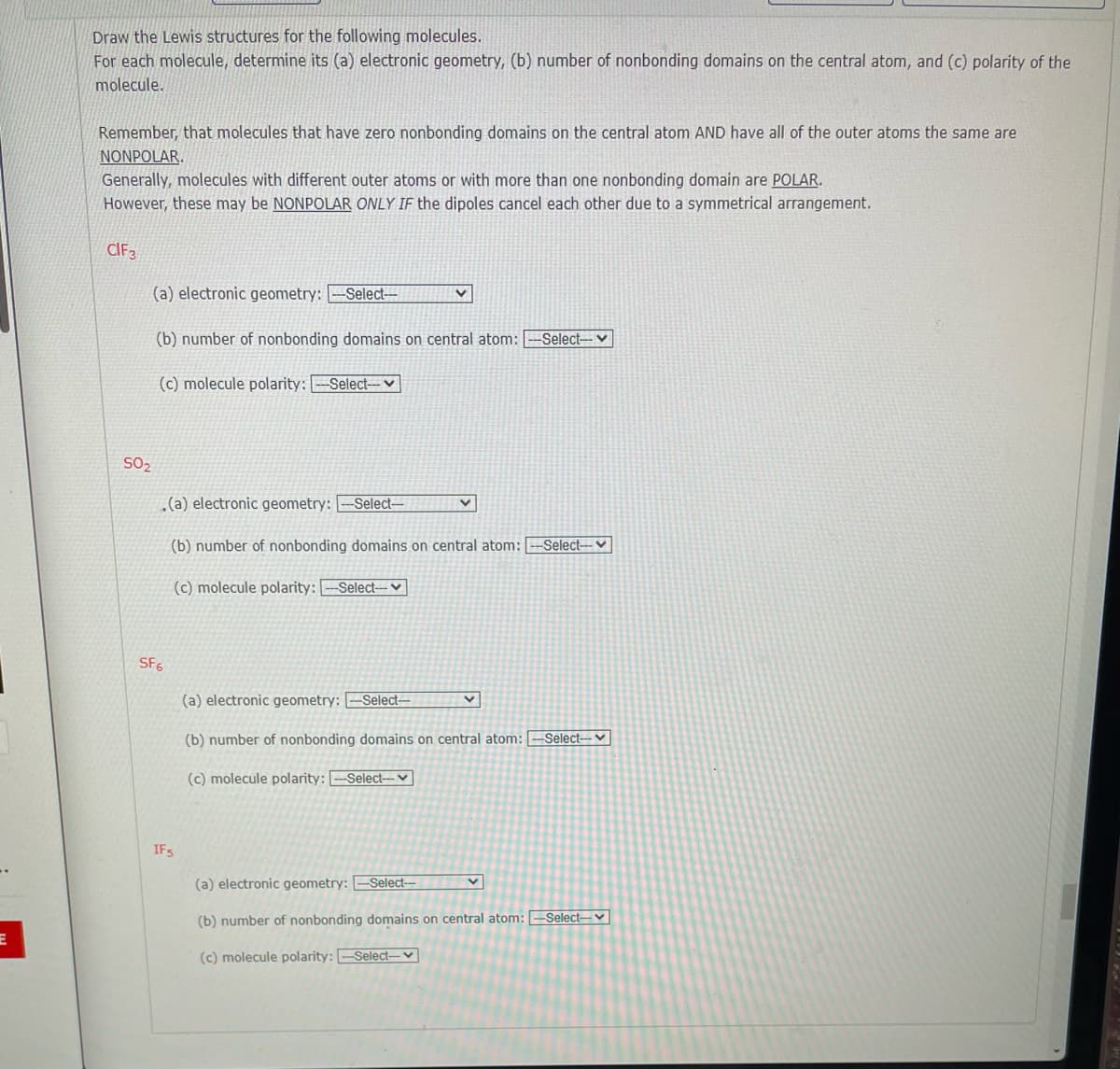
Transcribed Image Text:Draw the Lewis structures for the following molecules.
For each molecule, determine its (a) electronic geometry, (b) number of nonbonding domains on the central atom, and (c) polarity of the
molecule.
Remember, that molecules that have zero nonbonding domains on the central atom AND have all of the outer atoms the same are
NONPOLAR.
Generally, molecules with different outer atoms or with more than one nonbonding domain are POLAR.
However, these may be NONPOLAR ONLY IF the dipoles cancel each other due to a symmetrical arrangement.
CIF3
(a) electronic geometry: -Select--
(b) number of nonbonding domains on central atom:
-Select-v
(c) molecule polarity: -Select-
SO2
.(a) electronic geometry: --Select-
(b) number of nonbonding domains on central atom:
-Select---V
(c) molecule polarity: --Select--v
SF5
(a) electronic geometry: [-Select-
(b) number of nonbonding domains on central atom: --Select--- ♥
(c) molecule polarity: --Select-- v
IFS
(a) electronic geometry: -Select--
(b) number of nonbonding domains on central atom: --Select--V
(c) molecule polarity: -Select- v
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,