What are the values of q, w, AU, AH, AS, ASsurr, and ASuniv for the following constant pressure process for a system containing 0.203 moles of CH3OH ? CH3OH(g, 112.0 °C, 1.00 atm) → CH3OH(I, 27.0 °C, 1.00 atm) Assume that CH3OH(g) behaves as an ideal gas and the volume of CH3OH(1) is much less than that of CH3OH(g). Also, assume that the temperature of the surroundings is 27.0 °C. Data: Molar heat capacity for CH3OH(g), Cp.m = 44.1 J K-1 mol-1 Molar heat capacity for CH3OH(1), Cp.m = 81.1 J K-1 mol-1 Enthalpy of vaporization, AvapH = 35.2 kJ mol¬1 at 64.7 °C and 1.00 atm Enter answers that are accurate to three (3) or more significant figures.
What are the values of q, w, AU, AH, AS, ASsurr, and ASuniv for the following constant pressure process for a system containing 0.203 moles of CH3OH ? CH3OH(g, 112.0 °C, 1.00 atm) → CH3OH(I, 27.0 °C, 1.00 atm) Assume that CH3OH(g) behaves as an ideal gas and the volume of CH3OH(1) is much less than that of CH3OH(g). Also, assume that the temperature of the surroundings is 27.0 °C. Data: Molar heat capacity for CH3OH(g), Cp.m = 44.1 J K-1 mol-1 Molar heat capacity for CH3OH(1), Cp.m = 81.1 J K-1 mol-1 Enthalpy of vaporization, AvapH = 35.2 kJ mol¬1 at 64.7 °C and 1.00 atm Enter answers that are accurate to three (3) or more significant figures.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.39E: A refrigerator contains approximately 17cubic feet, or about 480 liters, of air. Assuming it acts as...
Related questions
Question
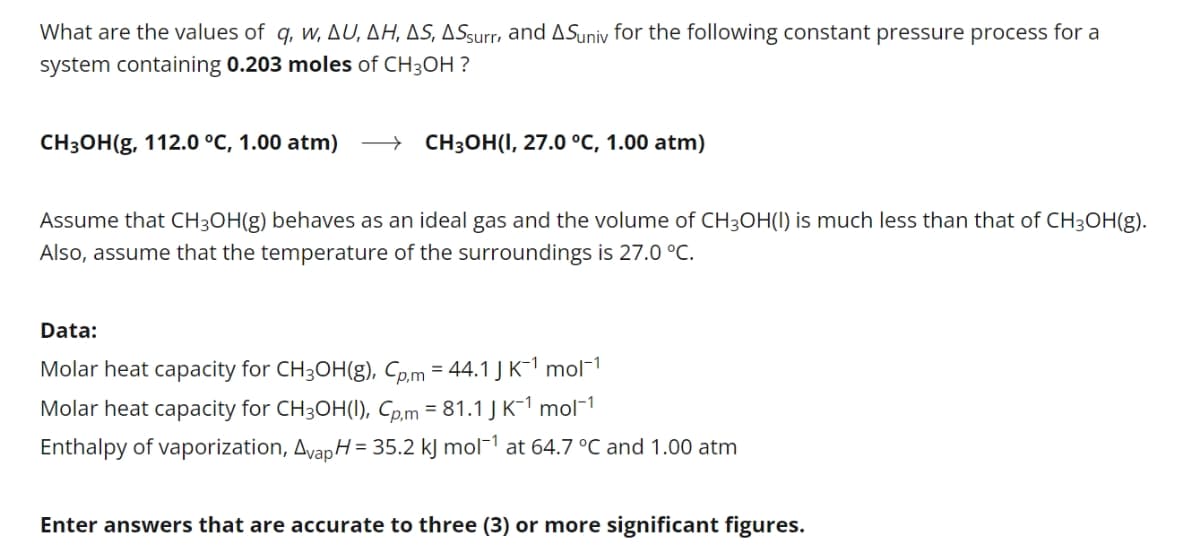
Transcribed Image Text:What are the values of q, w, AU, AH, AS, ASsurr, and ASuniv for the following constant pressure process for a
system containing 0.203 moles of CH3OH ?
CH3OH(g, 112.0 °C, 1.00 atm)
CH3OH(I, 27.0 °C, 1.00 atm)
Assume that CH3OH(g) behaves as an ideal gas and the volume of CH3OH(I) is much less than that of CH3OH(g).
Also, assume that the temperature of the surroundings is 27.0 °C.
Data:
Molar heat capacity for CH3OH(g), Cp.m = 44.1J K-1 mol¬1
Molar heat capacity for CH3OH(1), Cp.m = 81.1 J K-1 mol-1
Enthalpy of vaporization, Avap H= 35.2 kJ mol¯1 at 64.7 °C and 1.00 atm
Enter answers that are accurate to three (3) or more significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning