What can be concluded from the diffraction pattern generated by a single electron fired in the double slit experiment? Electrons can act like macroscopic particles. Electrons are smaller than photons. Pairs of electrons are needed to generate an interference pattern. Single electrons interfere with themselves.
What can be concluded from the diffraction pattern generated by a single electron fired in the double slit experiment? Electrons can act like macroscopic particles. Electrons are smaller than photons. Pairs of electrons are needed to generate an interference pattern. Single electrons interfere with themselves.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 50AP
Related questions
Question
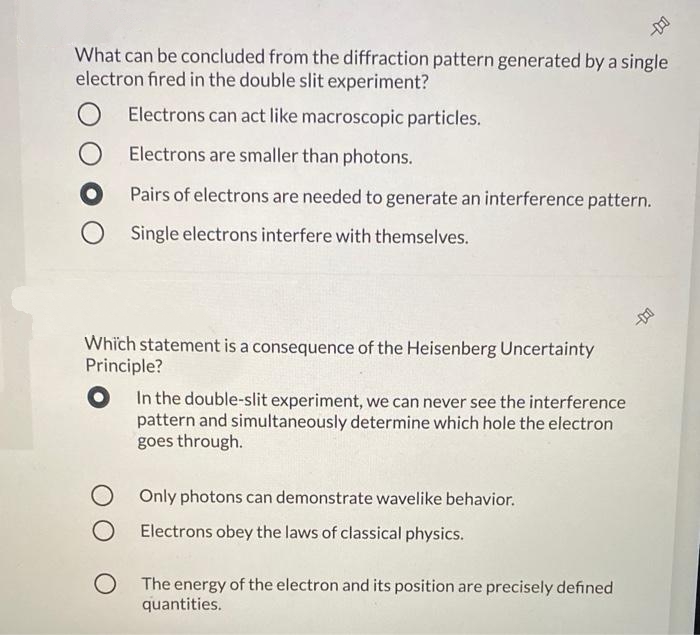
Transcribed Image Text:What can be concluded from the diffraction pattern generated by a single
electron fired in the double slit experiment?
Electrons can act like macroscopic particles.
Electrons are smaller than photons.
Pairs of electrons are needed to generate an interference pattern.
O Single electrons interfere with themselves.
Which statement is a consequence of the Heisenberg Uncertainty
Principle?
In the double-slit experiment, we can never see the interference
pattern and simultaneously determine which hole the electron
goes through.
Only photons can demonstrate wavelike behavior.
O Electrons obey the laws of classical physics.
O The energy of the electron and its position are precisely defined
quantities.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning