In general, metals will electrons, while nonmetals will electrons to get to their full octet. The closer elements are to completing their octet the more they will be. This means that the two most reactive groups on the periodic table are halogens and and the least reactive group on the periodic table is :: Noble Gases :: gain :: reactive :: Alkali Metals :: lose
In general, metals will electrons, while nonmetals will electrons to get to their full octet. The closer elements are to completing their octet the more they will be. This means that the two most reactive groups on the periodic table are halogens and and the least reactive group on the periodic table is :: Noble Gases :: gain :: reactive :: Alkali Metals :: lose
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 1E: Write the electronic configuration for the ions of the third period element that form monoatomic...
Related questions
Question
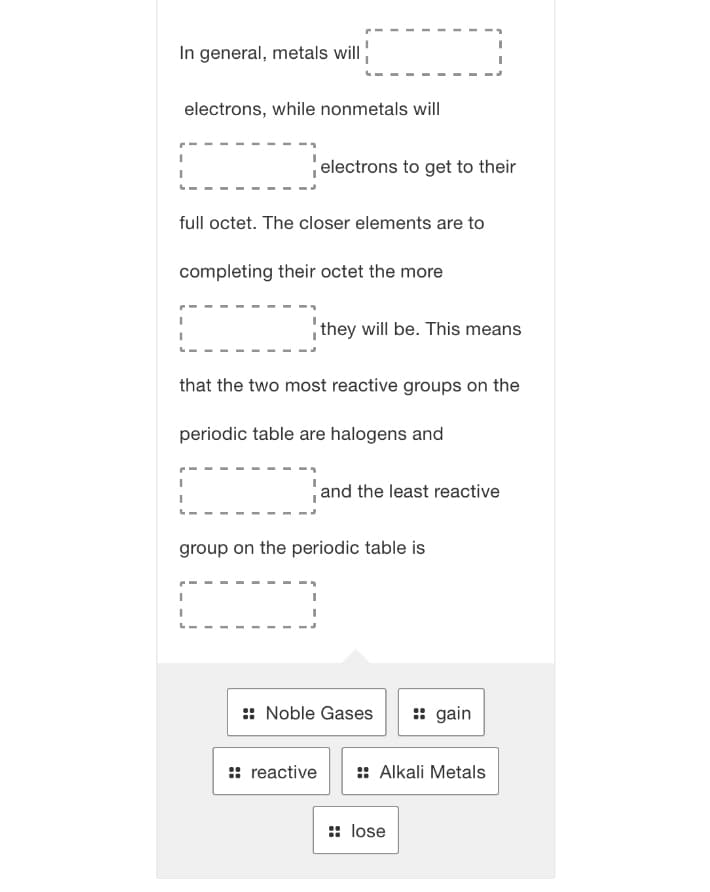
Transcribed Image Text:In general, metals will
electrons, while nonmetals will
electrons to get to their
full octet. The closer elements are to
completing their octet the more
they will be. This means
that the two most reactive groups on the
periodic table are halogens and
and the least reactive
group on the periodic table is
:: Noble Gases
: gain
:: reactive
:: Alkali Metals
:: lose
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning