Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 43QAP: Consider the equation: 2A+B5C. If 10.0 g of A reacts with 5.00 g of B. how is the limiting reactant...
Related questions
Question
100%
what is b c and d
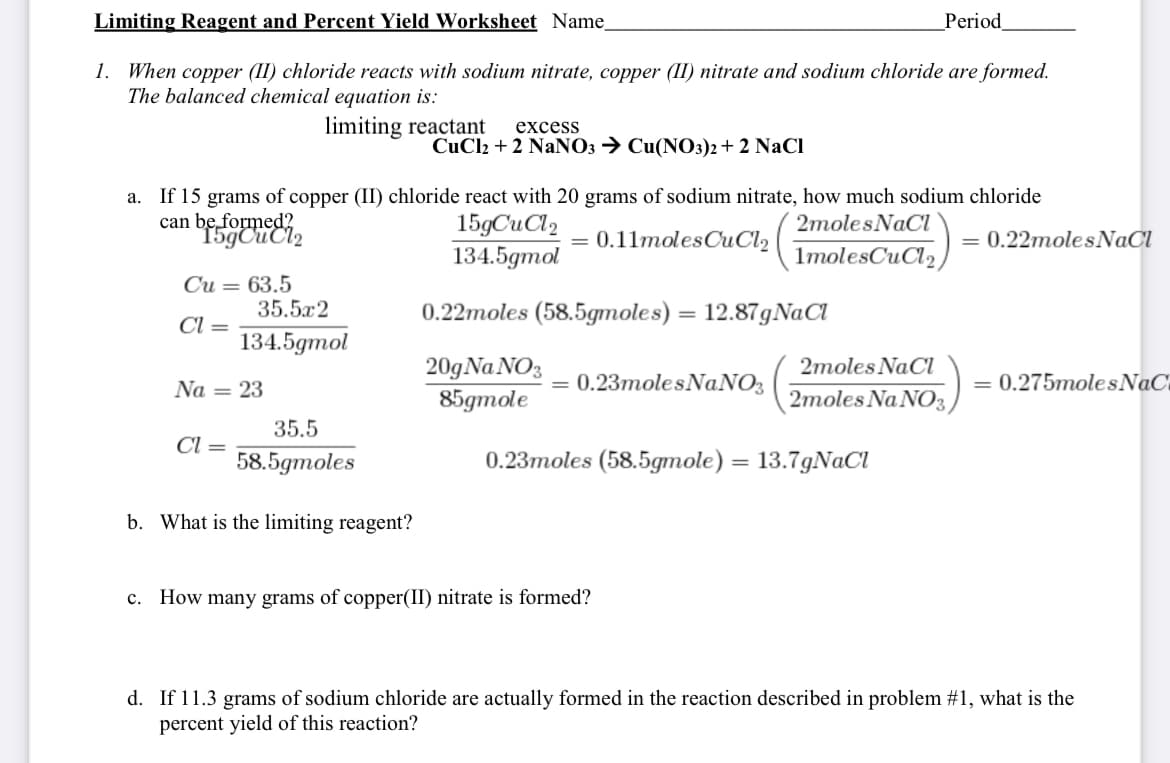
Transcribed Image Text:Limiting Reagent and Percent Yield Worksheet Name
_Period
1. When copper (1I) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed.
The balanced chemical equation is:
limiting reactant
excess
CuCl2 + 2 NaNO3 → Cu(NO3)2+ 2 NaCl
If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride
can beformeei,
a.
15gCuCl2
134.5gmol
2molesNaCl
0.11molesCuCl2
0.22molesNaCl
1molesCuCl2
Си — 63.5
35.5x2
0.22moles (58.5gmoles) = 12.87gNaCl
%3D
Cl =
134.5gmol
20gNa NO3
85gтole
2moles NaCl
Na = 23
0.23molesNaNO3
= 0.275molesNaC
2moles Na NO3,
35.5
Cl =
58.5gтoles
0.23тoles (58.5gтole) — 13.7gNaCl
%3D
b. What is the limiting reagent?
c. How many grams of copper(II) nitrate is formed?
d. If 11.3 grams of sodium chloride are actually formed in the reaction described in problem #1, what is the
percent yield of this reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax