Chapter18: Introduction To Electrochemistry
Section: Chapter Questions
Problem 18.13QAP
Related questions
Question
100%
20 round 2
Transcribed Image Text:19
20
21
Molten NaCl
The particle diagrams above represent NaCl(1) and its decomposition into Na(l) and Cl, (g) in an electrochemical cell after voltage is applied. Which of the following statements about the
thermodynamic favorability of the decomposition of NaCl(1) is supported by the particle diagrams and why?
The decomposition is thermodynamically favored because the transfer of electrons to and from the ions occurs at the electrodes.
The decomposition is thermodynamically favored because the formation of a gaseous product results in an increase in entropy.
C
The decomposition is not thermodynamically favored because the pure elements form only after electrical energy is supplied.
The decomposition is not thermodynamically favored because there is a decrease in the number of particles as the reaction proceeds.
US
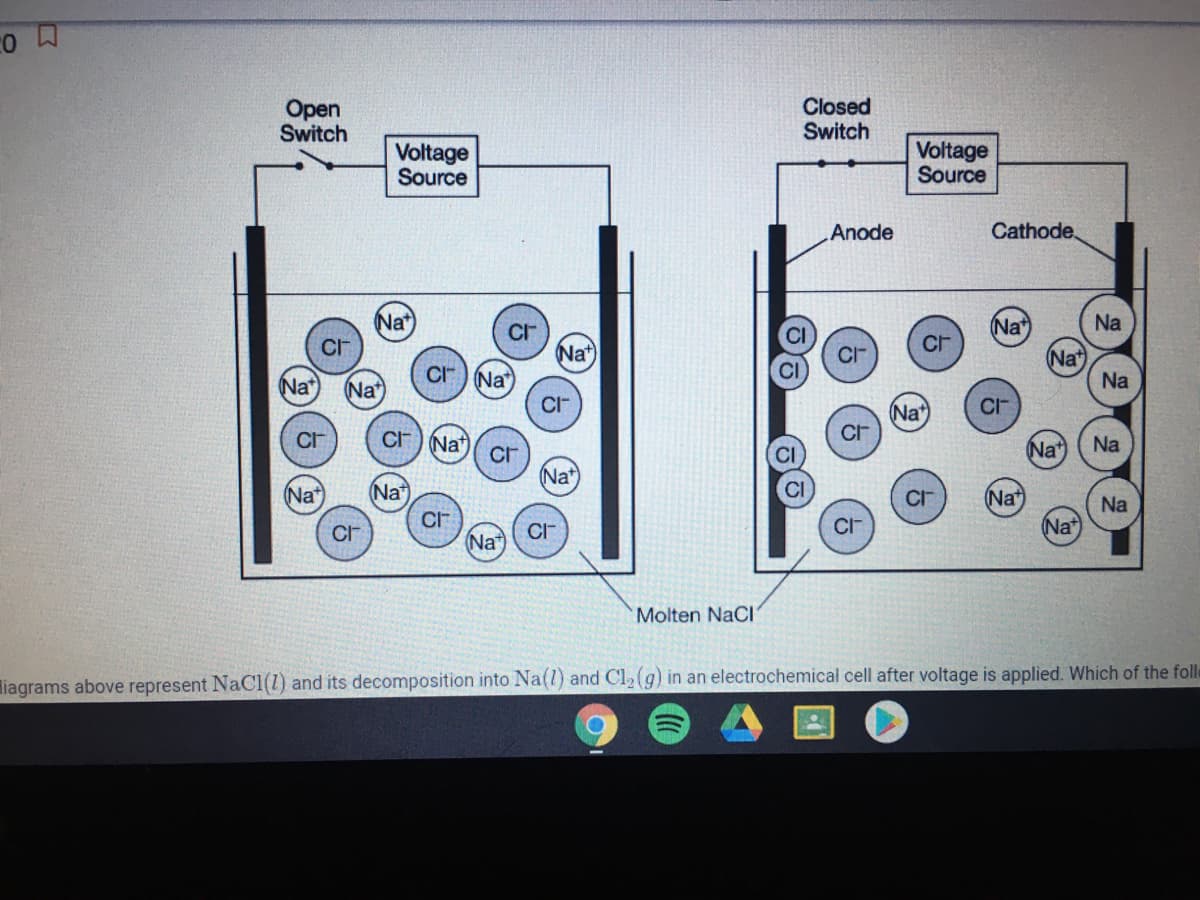
Transcribed Image Text:Open
Switch
Closed
Switch
Voltage
Source
Voltage
Source
Anode
Cathode,
Na
Na
Na
Nat
Na
Na
Na
Na
(Na
Nat
CF) Na
Nat
Na
cr
(Nat
Na*)
Na
(Na
Na
Na
CI
(Na
Molten NaCl
liagrams above represent NaCl(1) and its decomposition into Na(l) and Cl2(g) in an electrochemical cell after voltage is applied. Which of the fole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning