What is the CNN bond angle for the compound with the formula 7, (CH3)2CNNH2? (a) ~180° (b) -120° (c) -90° (d) -110° (e) -60° 8) What is the predicted shape, bond angle, and hybridization for +CH3? (a) trigonal planar, 120°, sp2 (b) trigonal planar, 120°, sp3 (c) trigonal planar, 109.5°, sp2 (d) trigonal pyramidal, 120°, sp2 (e) trigonal pyramidal, 109.5°, sp?
What is the CNN bond angle for the compound with the formula 7, (CH3)2CNNH2? (a) ~180° (b) -120° (c) -90° (d) -110° (e) -60° 8) What is the predicted shape, bond angle, and hybridization for +CH3? (a) trigonal planar, 120°, sp2 (b) trigonal planar, 120°, sp3 (c) trigonal planar, 109.5°, sp2 (d) trigonal pyramidal, 120°, sp2 (e) trigonal pyramidal, 109.5°, sp?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 39GQ: Phosphoserine is a less-common amino acid. (a) Identify the hybridizations of atoms I through 5. (b)...
Related questions
Question
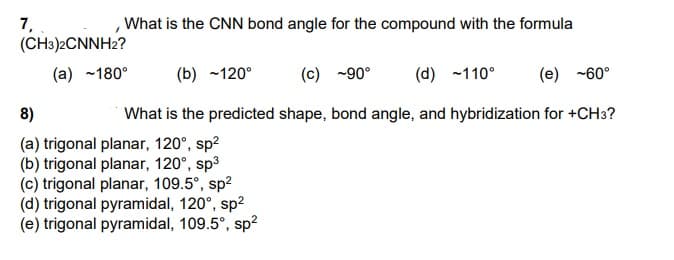
Transcribed Image Text:7,
(CH3)2CNNH2?
What is the CNN bond angle for the compound with the formula
(a) ~180°
(b) ~120°
(c) -90°
(d) -110°
(e) -60°
8)
What is the predicted shape, bond angle, and hybridization for +CH3?
(a) trigonal planar, 120°, sp2
(b) trigonal planar, 120°, sp3
(c) trigonal planar, 109.5°, sp2
(d) trigonal pyramidal, 120°, sp?
(e) trigonal pyramidal, 109.5°, sp?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning