What is the correct chemical equation when a solution of FeCl3 (aq) is mixed with a solution of Pb(NO3)2 (aq)? A CATION High iSolubility (aq) Low Solubility (s) Rb 181 ¹995- Cl, Br, I Halides Most no reaction Ag", Pb²", Tl, Hg²+, Hg", Cu F Group 1, NH, Group 2 Db Sg Solubility Table Most FeCl3 (aq)) + Pb(NO3)2 (aq) FeCl3 (aq)) + Pb(NO3)2 (aq) 2FeCl3 (aq)) + 3Pb(NO3)2 (aq) FeCl3 (aq)) + Pb(NO3)2 (aq) FeCl3 (aq)) + Pb(NO3)2 (aq) ANIONS OH Group 1, NHƯ, Sơn, Ba², Tl- All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in water. 241 Most SO4²- Periodic Table of the Elements Z11 Most 112** Ag", Pb²+, Ca², Ba, Sr²., Ra² 585 3FL3 Rg Cn CO₂, C₂H₂O₂ NOJ PO³, SO3²- Group 1, Most All NH₂ Most ²0¹ Ag Cd In Sn Fe(NO3)2 (aq) + PbCl3 (s) assz Ag None 082 Fe(NO3)3 (s) + PbCl₂ (s) Fe(NO3)2 (aq) + PbCl3 (aq) →→2Fe(NO3)3 (aq) + 3PbCl₂ (s) Fe(NO3)2 (s) + PbCl3 (s) Pb Bi Po Pm Gd Dy "Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Se Br Kr FIUup Lv F
What is the correct chemical equation when a solution of FeCl3 (aq) is mixed with a solution of Pb(NO3)2 (aq)? A CATION High iSolubility (aq) Low Solubility (s) Rb 181 ¹995- Cl, Br, I Halides Most no reaction Ag", Pb²", Tl, Hg²+, Hg", Cu F Group 1, NH, Group 2 Db Sg Solubility Table Most FeCl3 (aq)) + Pb(NO3)2 (aq) FeCl3 (aq)) + Pb(NO3)2 (aq) 2FeCl3 (aq)) + 3Pb(NO3)2 (aq) FeCl3 (aq)) + Pb(NO3)2 (aq) FeCl3 (aq)) + Pb(NO3)2 (aq) ANIONS OH Group 1, NHƯ, Sơn, Ba², Tl- All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in water. 241 Most SO4²- Periodic Table of the Elements Z11 Most 112** Ag", Pb²+, Ca², Ba, Sr²., Ra² 585 3FL3 Rg Cn CO₂, C₂H₂O₂ NOJ PO³, SO3²- Group 1, Most All NH₂ Most ²0¹ Ag Cd In Sn Fe(NO3)2 (aq) + PbCl3 (s) assz Ag None 082 Fe(NO3)3 (s) + PbCl₂ (s) Fe(NO3)2 (aq) + PbCl3 (aq) →→2Fe(NO3)3 (aq) + 3PbCl₂ (s) Fe(NO3)2 (s) + PbCl3 (s) Pb Bi Po Pm Gd Dy "Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Se Br Kr FIUup Lv F
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 60QAP: Gold metal will dissolve only in aqua regia, a mixture of concentrated hydrochloric acid and...
Related questions
Question
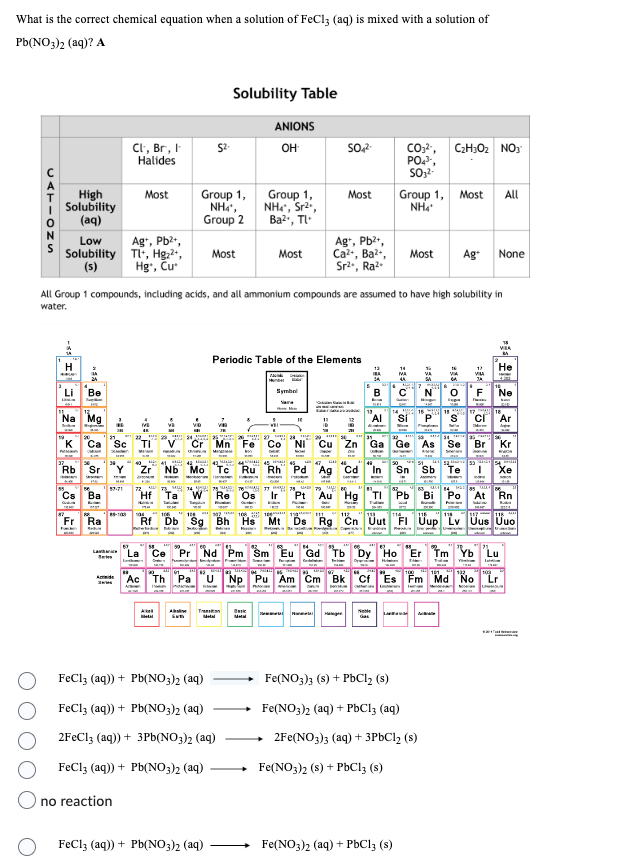
Transcribed Image Text:What is the correct chemical equation when a solution of FeCl3 (aq) is mixed with a solution of
Pb(NO3)2 (aq)? A
CATIONS
High
Solubility
(aq)
Low
Solubility
(s)
Li
"Na Mg
Be
Rb
"Cs Ba
Fr Ra
Lav
Act
Cl, Br, I
Halides
Most
Ag+, Pb²+,
Tl+, Hg₂²+,
Hg, Cu
no reaction
Group 1,
Nhì,
Group 2
Solubility Table
$2.
Most
All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in
water.
T
ANIONS
OH
FeCl3 (aq)) + Pb(NO3)2 (aq)
Group 1,
NH4*, Sr²,
Ba²+, Tl
Most
FeCl3 (aq)) + Pb(NO3)2 (aq)
FeCl3 (aq)) + Pb(NO3)2 (aq)
2FeCl3 (aq)) + 3Pb(NO3)2 (aq)
FeCl3 (aq)) + Pb(NO3)2 (aq)
24 25 26 27 28
Cr Mn Fe
Co
Periodic Table of the Elements
He
Symbol
Basic
10
SO4²-
Most
Ag+, Pb²+,
Ca²+, Ba²+,
Sr²., Ra².
Ti
Zr Nb Mo Tc Ru Rh Pd Ag Cd
Cu
Na
PER
CO3²-, C₂H30₂ NO3
PO4³-,
SO₂²-
Group 1, Most All
NH4+
76
Re Os Ir Pt Au Hg Tl Pb
Noble
Most Ag+ None
Sb Te
Hf Ta
Bi Po At Rn
89-108104
Rf Db Sg Bh Hs Mt Ds Rg Cn Uut "FI Uup Lv Uus Uuo
He
B с N OF Ne
Al
14_402||16_218||19-2410|17_044|||18,
S
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho
"Yb Lu
"Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
LUM
Fe(NO3)2 (aq) + PbC13 (s)
17
VILA
Fe(NO3)3 (s) + PbCl₂ (s)
Fe(NO3)2 (aq) + PbCl3 (aq)
→2Fe(NO3)3 (aq) + 3PbCl₂ (s)
Fe(NO3)2 (s) + PbCl3 (s)
CI
34_35_
15
VIA
Se Br
O DĄ JAU
Xe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning