What is the ground-state electronic configuration of a magnesium cation (Mg²*)? A) ls°, 2s², 2p° B) 1s?, 2s², 2pº, 3s' C) 1s?, 2s², 2p°, 3s² D) 1s?, 2s?, 2p°, 3s², 3p² What is the ground-state electronic configuration of a chlorine anion (Cl¬)? A) 1s°, 2s², 2p° B) 1s°, 2s°, 2pº, 3s², 3p° C) ls°, 2s², 2p°, 3s², 3p° D) 1s°, 2s², 2p°, 3s², 3p*
What is the ground-state electronic configuration of a magnesium cation (Mg²*)? A) ls°, 2s², 2p° B) 1s?, 2s², 2pº, 3s' C) 1s?, 2s², 2p°, 3s² D) 1s?, 2s?, 2p°, 3s², 3p² What is the ground-state electronic configuration of a chlorine anion (Cl¬)? A) 1s°, 2s², 2p° B) 1s°, 2s°, 2pº, 3s², 3p° C) ls°, 2s², 2p°, 3s², 3p° D) 1s°, 2s², 2p°, 3s², 3p*
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 66QAP: Consider the following transitions 1. n=3 to n=1 2. n=2 to n=33. n=4 to n=34. n=3 to n=5(a) For...
Related questions
Question
please answer the 2 questions please
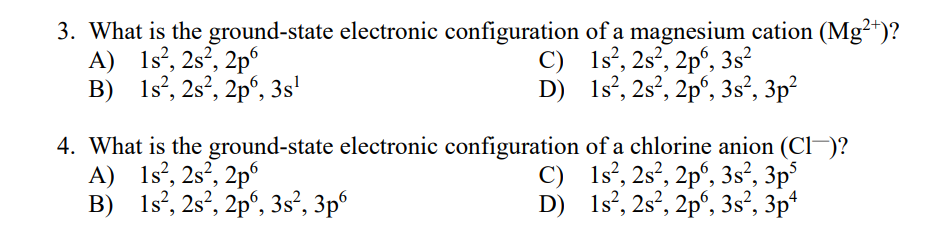
Transcribed Image Text:3. What is the ground-state electronic configuration of a magnesium cation (Mg²*)?
A) 1s?, 2s², 2p
B) 1s?, 2s², 2pº, 3s'
C) 1s°, 2s², 2p°, 3s²
D) 1s°, 2s?, 2p', 3s², 3p²
4. What is the ground-state electronic configuration of a chlorine anion (Cl-)?
A) 1s?, 2s², 2p°
B) 1s?, 2s?, 2p°, 3s², 3p°
C) 1s°, 2s², 2p°, 3s², 3p°
D) 1s', 2s?, 2pº, 3s², 3p*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning