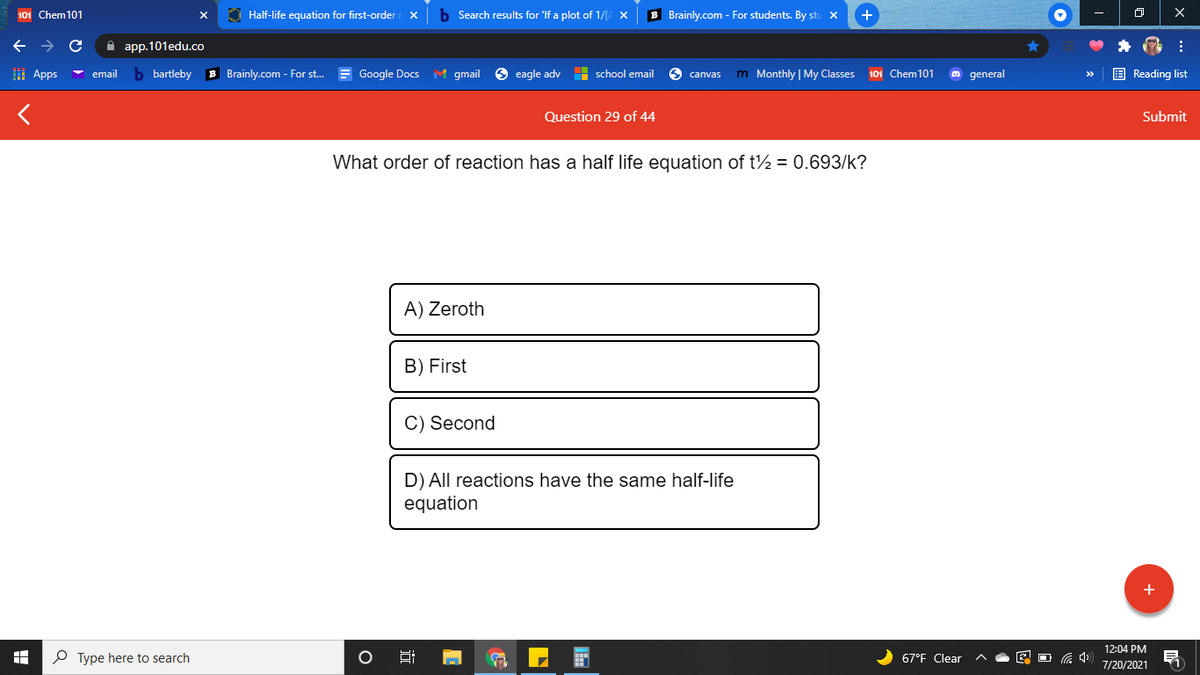
What order of reaction has a half life equation of t½ = 0.693/k? A) Zeroth B) First C) Second D) All reactions have the same half-life equation
What order of reaction has a half life equation of t½ = 0.693/k? A) Zeroth B) First C) Second D) All reactions have the same half-life equation
Chapter30: Kinetic Methods Of Analysis
Section: Chapter Questions
Problem 30.12QAP
Related questions
Question
What order of reaction has a half life equation of t½ = 0.693/k?
Transcribed Image Text:101 Chem101
Half-life equation for first-orderr X
b Search results for 'If a plot of 1/[ X
B Brainly.com - For students. By stu X
+
->
A app.101edu.co
I Apps
b bartleby
B Brainly.com - For st. = Google Docs M gmail
6 eagle adv
H school email
S canvas
m Monthly | My Classes
E Reading list
email
101 Chem101
general
Question 29 of 44
Submit
What order of reaction has a half life equation of t½ = 0.693/k?
A) Zeroth
B) First
C) Second
D) All reactions have the same half-life
equation
12:04 PM
P Type here to search
67°F Clear
O G 4)
7/20/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

