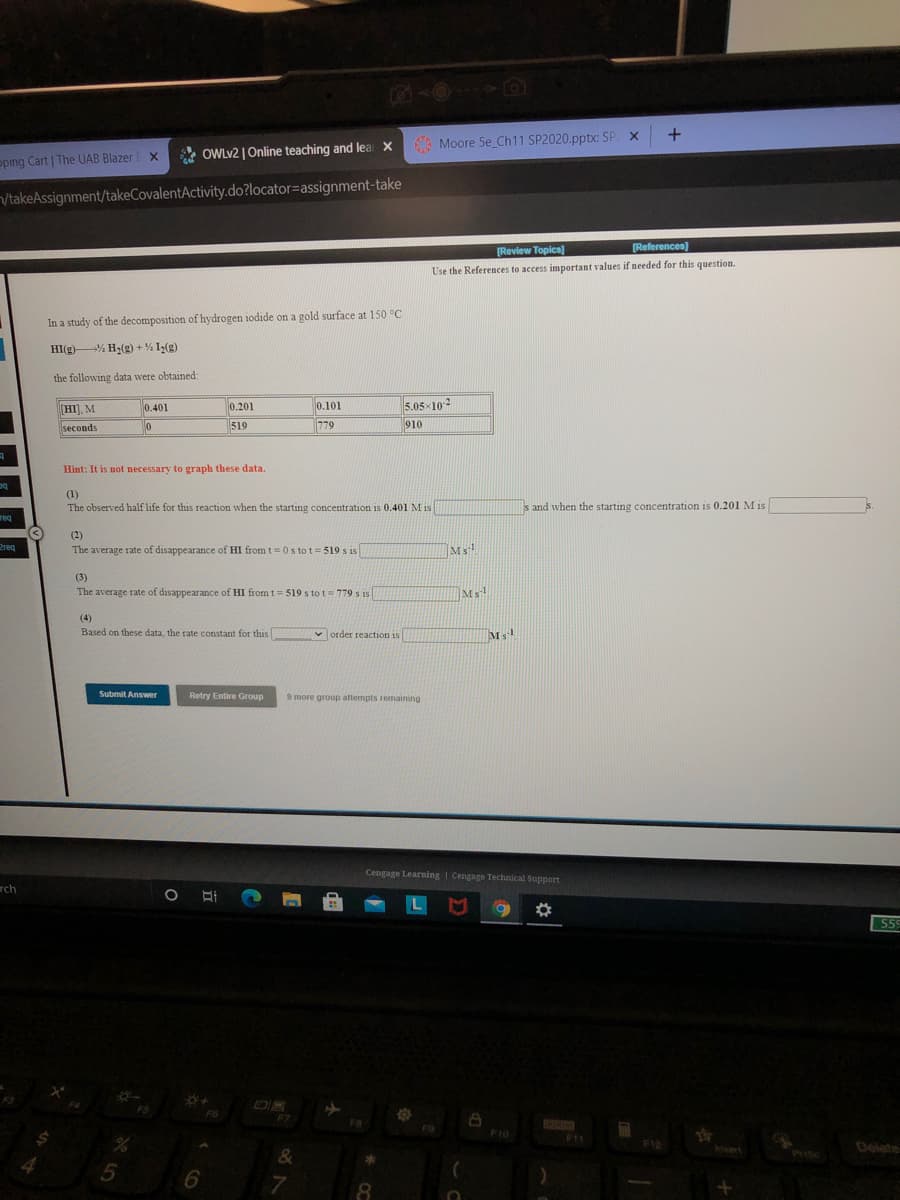
In a study of the decomposition of hydrogen iodide on a gold surface at 150 °C HI(g H2(g) + % I(g) the following data were obtained: (HI], M 0.401 0.201 0.101 5.05-10 seconds 519 779 910 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.401 M is and when the starting concentration is 0.201 M is (2) The average rate of disappearance of HI from t=0s to t= 519 s is (3) The average rate of disappearance of HI from t= 519 s to t= 779 s i Ms! (4) Based on these data, the rate constant for this v order reaction is Submit Answer Retry Entire Group 9more group attempts remaining
In a study of the decomposition of hydrogen iodide on a gold surface at 150 °C HI(g H2(g) + % I(g) the following data were obtained: (HI], M 0.401 0.201 0.101 5.05-10 seconds 519 779 910 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.401 M is and when the starting concentration is 0.201 M is (2) The average rate of disappearance of HI from t=0s to t= 519 s is (3) The average rate of disappearance of HI from t= 519 s to t= 779 s i Ms! (4) Based on these data, the rate constant for this v order reaction is Submit Answer Retry Entire Group 9more group attempts remaining
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter30: Capillary Electrophoresis, Electrochromatography, And Field-flow Fractionation
Section: Chapter Questions
Problem 30.9QAP
Related questions
Question
Transcribed Image Text:+
Moore 5e_Ch11 SP2020.pptx: SP X
* OWLV2 | Online teaching and lea X
oping Cart | The UAB Blazer x
VtakeAssignment/takeCovalentActivity.do?locator=assignment-take
[Review Topica)
[References)
Use the References to access important values if needed for this question.
In a study of the decomposition of hydrogen iodide on a gold surface at 150 °C
HI(g)% H2(g) + % I(g)
the following data were obtained:
HI), M
0.401
0.201
0.101
5.05x102
seconds
519
779
910
Hint: It is not necessary to graph these data.
(1)
The observed half life for this reaction when the starting concentration is 0.401 M is
s and when the starting concentration is 0.201 M is
req
(2)
2req
The average rate of disappearance of HI from t=0 s to t= 519 s is
Ms!
(3)
The average rate of disappearance of HI from t = 519 s to t= 779 s is
Ms!
(4)
Based on these data, the rate constant for this
v order reaction is
Submit Answer
Retry Entire Group
9 more group attempts remaining
Cengage Learning | Cengage Technical Support
Tch
55
F7
F8
F9
24
F10
F11
F12
Deiete
sert
&
Prd
4
6
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning