What will be the new boilng point of 297ml benzene (Kb = 2.53 °C.Kg/mol; Bp = 80.1°C) when mixed with 14g of naphthalene (MW = 128.17 g/mol) to make a non- eletrolyte solution? Density of benzene = 0.876 g/ml) Round you answer in 2 decimal places, unit is not required.
What will be the new boilng point of 297ml benzene (Kb = 2.53 °C.Kg/mol; Bp = 80.1°C) when mixed with 14g of naphthalene (MW = 128.17 g/mol) to make a non- eletrolyte solution? Density of benzene = 0.876 g/ml) Round you answer in 2 decimal places, unit is not required.
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.44E
Related questions
Question
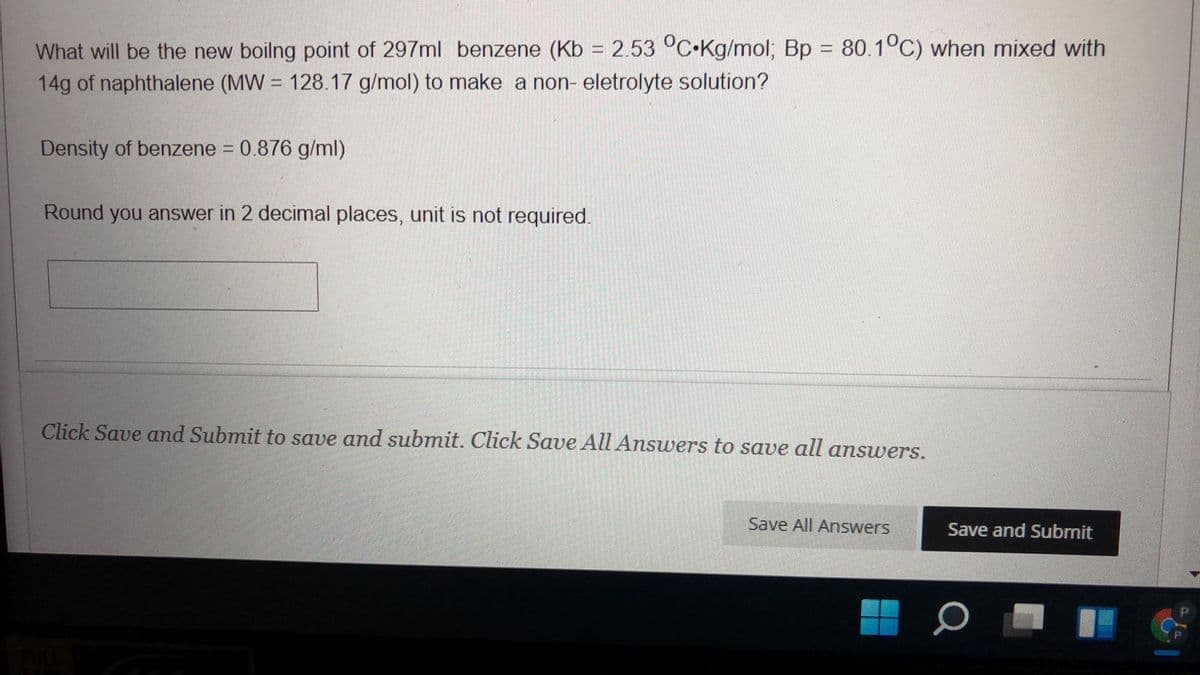
Transcribed Image Text:What will be the new boilng point of 297ml benzene (Kb = 2.53 °C•Kg/mol; Bp 80.1°C) when mixed with
14g of naphthalene (MW = 128.17 g/mol) to make a non- eletrolyte solution?
%3D
Density of benzene = 0.876 g/ml)
Round you answer in 2 decimal places, unit is not required.
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Save All Answers
Save and Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

