When a rotating disk electrode (RDE) is held at a high enough potential, the rate of the reaction is governed by the diffusion rate, the rate at which the analyte diffuses through the diffusion layer to the electrode. The thickness of this diffusion layer, 8, is calculated as 8 = 1.61 D¹36-1/21/6 where D is the diffusion coefficient (m²/s), v is the kinematic viscosity of the liquid (m²/s), and is the rotation rate (radians/s) of the electrode. Additionally, the current density, J (A/m²), is measured using the Levich equation, J = 0.62nFD2/3¹/2-1/6 Co where n is the number of electrons transferred in the half-reaction, F is the Faraday constant, and Co is the concentration of the electroactive species (mol/m³). Calculate 8, in micrometers, and the current density, J, for the reduction of 0.020 M T1³+ to Tl* at a gold electrode in 1 F HCl at 0.80 V vs. SHE at 2300 rpm, where D = 2.5 x 10-9 m²/s and v= 2.0 x 10-6 m²/s. 8= μm
When a rotating disk electrode (RDE) is held at a high enough potential, the rate of the reaction is governed by the diffusion rate, the rate at which the analyte diffuses through the diffusion layer to the electrode. The thickness of this diffusion layer, 8, is calculated as 8 = 1.61 D¹36-1/21/6 where D is the diffusion coefficient (m²/s), v is the kinematic viscosity of the liquid (m²/s), and is the rotation rate (radians/s) of the electrode. Additionally, the current density, J (A/m²), is measured using the Levich equation, J = 0.62nFD2/3¹/2-1/6 Co where n is the number of electrons transferred in the half-reaction, F is the Faraday constant, and Co is the concentration of the electroactive species (mol/m³). Calculate 8, in micrometers, and the current density, J, for the reduction of 0.020 M T1³+ to Tl* at a gold electrode in 1 F HCl at 0.80 V vs. SHE at 2300 rpm, where D = 2.5 x 10-9 m²/s and v= 2.0 x 10-6 m²/s. 8= μm
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter25: Voltammetry
Section: Chapter Questions
Problem 25.17QAP
Related questions
Question
Please help solve this question, will provide thumbs up if correct, Thank you.
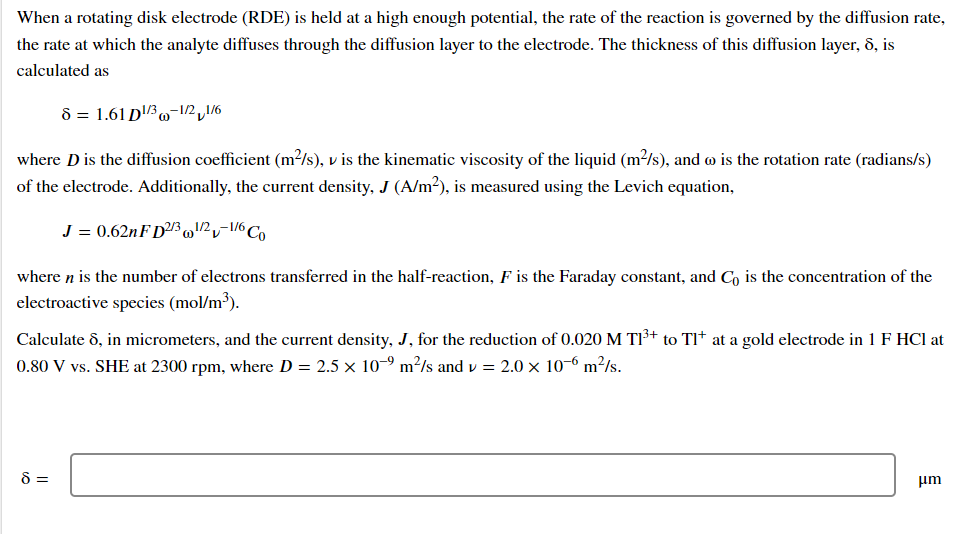
Transcribed Image Text:When a rotating disk electrode (RDE) is held at a high enough potential, the rate of the reaction is governed by the diffusion rate,
the rate at which the analyte diffuses through the diffusion layer to the electrode. The thickness of this diffusion layer, 8, is
calculated as
8 = 1.61 D¹/3-1/2,1/6
where D is the diffusion coefficient (m²/s), v is the kinematic viscosity of the liquid (m²/s), and wo is the rotation rate (radians/s)
of the electrode. Additionally, the current density, J (A/m²), is measured using the Levich equation,
J = 0.62nFD2/3¹/2-1/6 Co
where n is the number of electrons transferred in the half-reaction, F is the Faraday constant, and Co is the concentration of the
electroactive species (mol/m³).
Calculate 8, in micrometers, and the current density, J, for the reduction of 0.020 M T1³+ to Tl* at a gold electrode in 1 F HCl at
0.80 V vs. SHE at 2300 rpm, where D = 2.5 × 10-⁹ m²/s and v = 2.0 × 10-6 m²/s.
8 =
μm
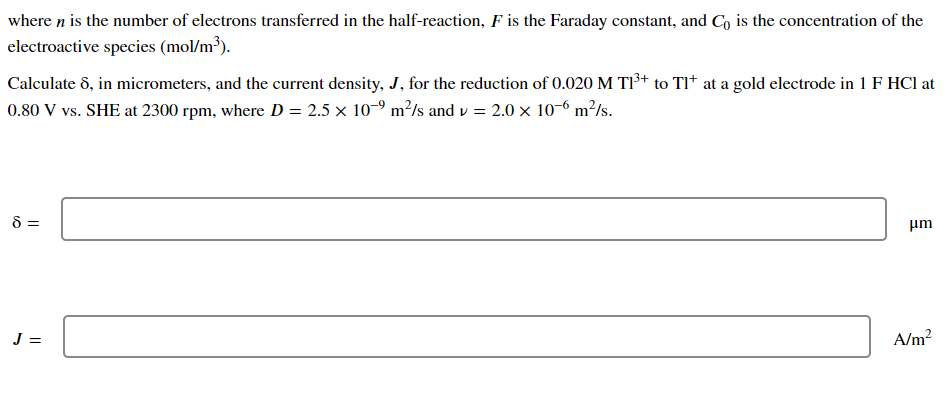
Transcribed Image Text:where n is the number of electrons transferred in the half-reaction, F is the Faraday constant, and Co is the concentration of the
electroactive species (mol/m³).
Calculate 8, in micrometers, and the current density, J, for the reduction of 0.020 M T1³+ to Tl* at a gold electrode in 1 F HCI at
0.80 V vs. SHE at 2300 rpm, where D = 2.5 x 10-9 m²/s and v= 2.0 x 10-6 m²/s.
8 =
J =
μm
A/m²
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

