When equal molar amounts of phthalic anhydride and 1,2,3-propanetriol are heated, they form an amorphous polyester. Under these conditions, polymerization is regio- selective for the primary hydroxyl groups of the triol. OH heat + HO. LOH a polyester Phthalic anhydride 1,2,3-Propanetriol (Glycerol)
When equal molar amounts of phthalic anhydride and 1,2,3-propanetriol are heated, they form an amorphous polyester. Under these conditions, polymerization is regio- selective for the primary hydroxyl groups of the triol. OH heat + HO. LOH a polyester Phthalic anhydride 1,2,3-Propanetriol (Glycerol)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter29: Organic Polymer Chemistry
Section: Chapter Questions
Problem 29.19P
Related questions
Question
The polyester can be mixed with additional phthalic anhydride
(0.5 mole of phthalic anhydride for each mole of 1,2,3-propanetriol in the original
polyester) to form a liquid resin. When this resin is heated, it forms a hard, insoluble thermosetting polyester called glyptal.
Q.) Propose a structure for the repeat unit in glyptal.
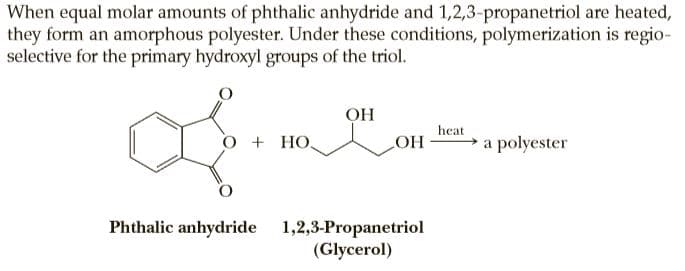
Transcribed Image Text:When equal molar amounts of phthalic anhydride and 1,2,3-propanetriol are heated,
they form an amorphous polyester. Under these conditions, polymerization is regio-
selective for the primary hydroxyl groups of the triol.
OH
heat
+ HO.
LOH a polyester
Phthalic anhydride 1,2,3-Propanetriol
(Glycerol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning