When NO() reacts with O;(g) according to the following teaction, 57.1 kJ of energy are evolved for each mole of NO(g) that reacts. Complete the following thermochemical equation 2NO() O()2NO,() AH-
When NO() reacts with O;(g) according to the following teaction, 57.1 kJ of energy are evolved for each mole of NO(g) that reacts. Complete the following thermochemical equation 2NO() O()2NO,() AH-
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.79PAE: A student performing a calorimetry experiment combined 100.0 ml. of 0.50 M HCI and 100.0 ml. of 0.50...
Related questions
Question
3
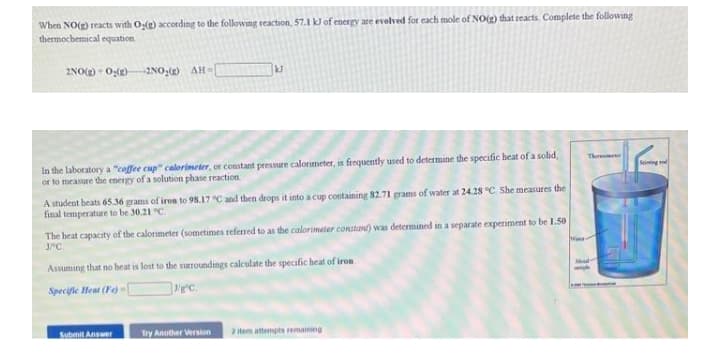
Transcribed Image Text:When NO() reacts with Og(g) according to the following reaction, 57.1 k of energy are evelved for each mole of NO(g) that reacts. Complete the following
thermochemical equation.
2NO() - 0:(2)
-2NO:() AH-
In the laboratory a "cofee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid,
or to measure the energy of a solution phase reaction
Therem
Seiming
A student beats 65.36 grams of iron to 98.17 C and then drops it into a cup containing 82.71 grams of water at 24.28 °C. She measures the
final temperature to be 30.21 "C.
The heat capacaty of the calorimeter (sometimes referned to as the calorimeter constant) was determined in a separate experiment to be 1.50
JPC
Assuming that no heat is lost to the surroundings calculate the specific heat of iron
Md-
Specifie Heat (Fe) -{
ec.
Submit Answer
Try Another Version
a tem attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning