When phenyl-5-bromopentanone is treated with triphenylphosphine, followed by base, a compound is produced whose formula is C11H12. Its 13C NMR spectrum has nine signals, six of which appear between 120 and 140 ppm and three of which appear below 50 ppm. For this reaction, draw the complete, detailed mechanism, as well as the major organic product. 1. P(C6H5)3 Br C11H12 2. NaOCH,CH3
When phenyl-5-bromopentanone is treated with triphenylphosphine, followed by base, a compound is produced whose formula is C11H12. Its 13C NMR spectrum has nine signals, six of which appear between 120 and 140 ppm and three of which appear below 50 ppm. For this reaction, draw the complete, detailed mechanism, as well as the major organic product. 1. P(C6H5)3 Br C11H12 2. NaOCH,CH3
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter24: Carboxylic Acids & Derivatives
Section: Chapter Questions
Problem 3E
Related questions
Question
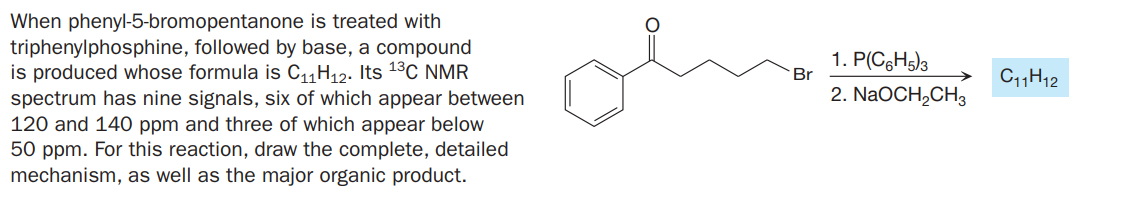
Transcribed Image Text:When phenyl-5-bromopentanone is treated with
triphenylphosphine, followed by base, a compound
is produced whose formula is C11H12. Its 13C NMR
spectrum has nine signals, six of which appear between
120 and 140 ppm and three of which appear below
50 ppm. For this reaction, draw the complete, detailed
mechanism, as well as the major organic product.
1. P(C6H5)3
Br
C11H12
2. NaOCH,CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning