When ß-carotene is oxidised in vivo, it breaks in half and forms two molecules of retinal (vitamin A), which is a precursor to the pigment in the retina responsible for vision. The conjugated system of retinal consists of eleven C atoms and one O atom; see the structure of the molecule here: H3C CH3 CH3 CH3 `H CH3 In the ground state of retinal, each level up to n = 6 is occupied by two electrons. Assuming an average inter-nuclear distance of 140 pm, use the particle-in-a-box model to calculate: a. the separation in energy between the ground state and first excited state in which one electron occupies the state with n = 7, and b. the frequency of the radiation required to produce a transition between these two states.
When ß-carotene is oxidised in vivo, it breaks in half and forms two molecules of retinal (vitamin A), which is a precursor to the pigment in the retina responsible for vision. The conjugated system of retinal consists of eleven C atoms and one O atom; see the structure of the molecule here: H3C CH3 CH3 CH3 `H CH3 In the ground state of retinal, each level up to n = 6 is occupied by two electrons. Assuming an average inter-nuclear distance of 140 pm, use the particle-in-a-box model to calculate: a. the separation in energy between the ground state and first excited state in which one electron occupies the state with n = 7, and b. the frequency of the radiation required to produce a transition between these two states.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter7: Molecular Structures
Section: Chapter Questions
Problem 66QRT
Related questions
Question
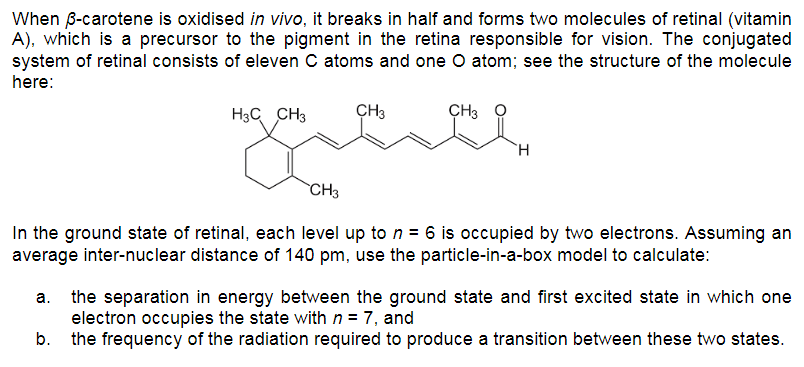
Transcribed Image Text:When B-carotene is oxidised in vivo, it breaks in half and forms two molecules of retinal (vitamin
A), which is a precursor to the pigment in the retina responsible for vision. The conjugated
system of retinal consists of eleven C atoms and one O atom; see the structure of the molecule
here:
H3C CH3
ÇH3
ÇH3
`CH3
In the ground state of retinal, each level up to n = 6 is occupied by two electrons. Assuming an
average inter-nuclear distance of 140 pm, use the particle-in-a-box model to calculate:
a. the separation in energy between the ground state and first excited state in which one
electron occupies the state with n = 7, and
b. the frequency of the radiation required to produce a transition between these two states.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,