When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? CrO42 Cr3+ Sn2++ Sn+ Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction?
When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? CrO42 Cr3+ Sn2++ Sn+ Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) How many electrons are transferred in this reaction?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter18: Oxidation–reduction Reactions And Electrochemistry
Section: Chapter Questions
Problem 84AP: . For each of the following unbalanced oxidation-reduction chemical equations, balance the equation...
Related questions
Question
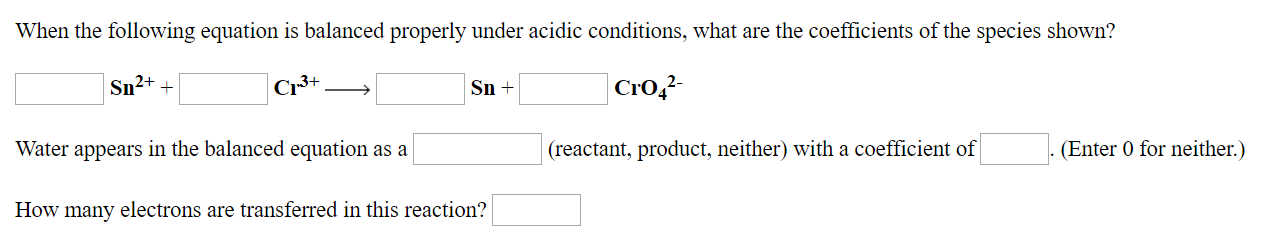
Transcribed Image Text:When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?
CrO42
Cr3+
Sn2++
Sn+
Water appears in the balanced equation as a
(reactant, product, neither) with a coefficient of
(Enter 0 for neither.)
How many electrons are transferred in this reaction?
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 8 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

