When the Hg2+ concentration is 1.01 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.577V. What is the Al3+ concentration? 3Hg2+(aq) + 2Al(s)3Hg(l) + 2Al3+(aq)
When the Hg2+ concentration is 1.01 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.577V. What is the Al3+ concentration? 3Hg2+(aq) + 2Al(s)3Hg(l) + 2Al3+(aq)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 68QAP: Calculate the voltages of the following cells at 25°C and under the following conditions: (a)...
Related questions
Question
When the Hg2+ concentration is 1.01 M, the observed cell potential at 298K for an
3Hg2+(aq) + 2Al(s)3Hg(l) + 2Al3+(aq)
Answer: ? M
(ps the exponential thing doesn't work ex: 1.35x^10-4M has to be decimal I think ty!)
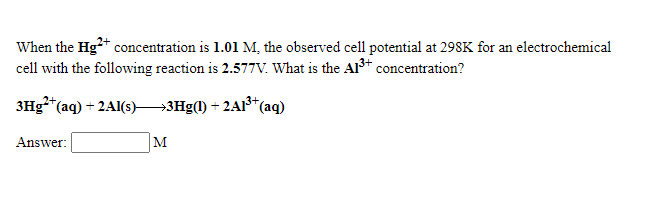
Transcribed Image Text:When the Hg* concentration is 1.01 M, the observed cell potential at 298K for an electrochemical
cell with the following reaction is 2.577V. What is the Al3+ concentration?
3Hg**(aq) + 2A1(s)3Hg(1) + 2A1³+*(aq)
Answer:
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning